A Tale of Recurrent Strokes due to Hypereosinophilic Syndrome
Tushar Premraj Raut, Gaurav Baheti, Sonal Raut, Dhiraj Bhattad, Pranay Jain and Ankur Jain
DOI10.21767/2471-299X.1000058
Tushar Premraj Raut*, Gaurav Baheti, Sonal Raut, Dhiraj Bhattad, Pranay Jain and Ankur Jain
Department of Neurology, Seven Hills Hospital, Andheri East Mumbai, India
- *Corresponding Author:
- Raut TP
Department of Neurology, Consultant Neurology
Seven Hills Hospital, Andheri East Mumbai, India
Tel: +91-9930087434
E-mail: tushar.27r@gmail.com
Received date: September 28, 2017; Accepted date: October 24, 2017; Published date: November 06, 2017
Citation: Raut TP, Baheti G, Raut S, Bhattad D, Jain P, et al. (2017) A Tale of Recurrent Strokes due to Hypereosinophilic Syndrome. Med Clin Rev. 3:16. doi: 10.21767/2471-299X.1000058
Copyright: © 2017 Raut TP, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Recurrent strokes are a huge cause for concern as with each stroke there is further accumulation of disability. There are various vascular risk factors responsible for recurrent stroke. However patients without any traditional risk factor or recurrence of stroke despite of best medical management should be screened for rare but important causes for stroke recurrence, once such cause being hypereosiniophilia. Although rare, Hypereosinophilic syndrome (HES) can cause recurrent ischemic stroke. Underlying pathogenesis of hypereosinophilic syndrome could be heterogeneous but cardioembolism is the most common etiology for stroke. Also there is eosinophilia mediated cytotoxicity and release of proinflammatory cytokines contributing to tissue damage and multiple organ involvement and recurrent stroke. Here the authors describe a case of a middle aged gentleman presenting with recurrent strokes over a period of 3 years. He had other risk factors like dyslipidemia and despite of dual antiplatelet therapy and statins he continued to get recurrent strokes with increasing severity. Extensive laboratory workup ruled out other etiologies for the strokes and the only possible causative factor which could be identified was hypereosinophilia. Work up for hypereosiniophilia was negative. After starting steroids and normalisation of eosinophil count, there was no further recurrence of stroke.
Keywords
Recurrent stroke; Hypereosinophilic syndrome (HES); Dyslipidemia; Antiplatelet
Introduction
About 1/3rd of the strokes tend to recur characterised by a neurological deficit lasting atleast 24 hours or more in same or different vascular territory. With every single stroke there is neuronal loss and deterioration in overall functional status which can be hazardous as well as life threatening. The best way to prevent stroke recurrence is to target the risk factor and treat the etiology for stroke. There are many conventional vascular risk factors. However patients with no typical risk factors and still getting a recurrent stroke are a cause for major concern. One such cause being hypereosiniophilia. Eosinophilia defined by a rise in eosinophil count in peripheral blood more than 6% or 450 per cu.mm. ‘Hypereosinophilic Syndrome’ (HES) refers to a group of disorders characterized by sustained overproduction of eosinophils with multiple organ involvement due to direct cellular toxicity and inflammatory mediators released by eosinophils. Neurological involvement is very common presenting as recurrent strokes, encephalopathy and neuropathy. The authors here report a case of a gentleman presenting with recurrent strokes with multiorgan involvement due to hypereosinophilia. HES have substantial clinical heterogeneity and variable prognosis, but usually responds well to steroid therapy.
Case Report
A 53-year-old gentleman known case of dyslipidemia well controlled on statins and having past history of multiple strokes presented with acute onset slurring of speech and left sided weakness since morning on waking up. Within few minutes weakness recovered however speech was slurred and facial weakness was present. On clinical examination vitals were stable. General examination was unremarkable. On neurological evaluation patient had dysarthria, left supranuclear facial palsy and left pronator drift. Patient was already on aspirin, clopidogrel and rosuvastatin and had presented outside the window period. His national insititue of health stroke scale (nihss) score was 3. He had past history of recurrent strokes. He also complained of dry cough from 6 months and non-specific symptoms like fatigue, myalgia. On leading question there was some confusion and apathy and slowing of activites as per relatives since last few months. Patient was also a known case of hypothyroidism on eltroxin supplementation. He had surgery in the past for sinonasal polyposis.
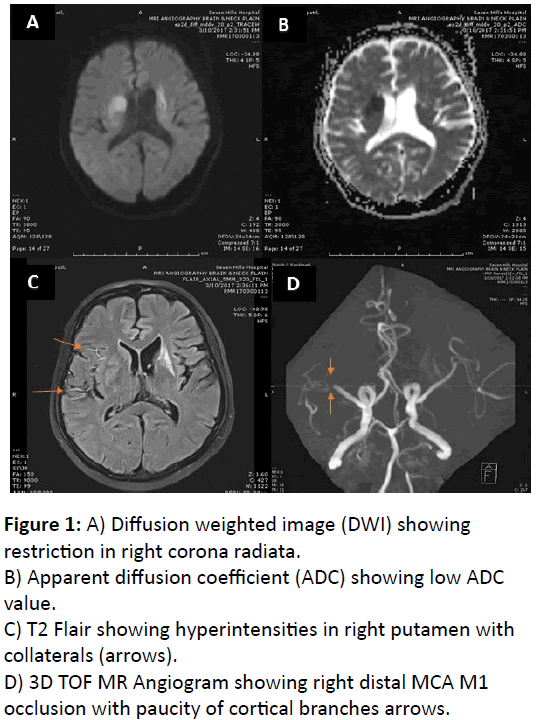
His routine hematological and biochemical investigations were sent. Brain magnetic resonance imaging (MRI) study showed acute infarct in right corpus striatum with bilateral multiple chronic lacunar infarcts. There was right distal middle cerebral artery (mca) m1 segment occlusion with paucity of cortical branches arrows with collaterals seen on flair (Figure 1A-D). Etiological workup for stroke, including lipid profile, electrocardiogram, Carotid doppler, transthoracic echocardiogram (TTE), and tests for hypercoagulable state including thrombophilia profile was negative. Mild leukocytosis (11,200/mm3) and elevated eosinophil count (22%) were noted with absolute eosiniophil count of 3200/ cu.mm. Serum creatinine was raised (2.1 mg%). urine routine examination suggestive of microalbuminuria without any casts/crystals. High resolution computed tomography (HRCT) chest revealed nonspecific interstitial pneumonia like pattern. Pulmonary function test was normal.
Figure 1: A) Diffusion weighted image (DWI) showing restriction in right corona radiata.
B) Apparent diffusion coefficient (ADC) showing low ADC value.
C) T2 Flair showing hyperintensities in right putamen with collaterals (arrows).
D) 3D TOF MR Angiogram showing right distal MCA M1 occlusion with paucity of cortical branches arrows.
He was extensively evaluated in the past for recurrent stroke, including transesosphageal echocardiogram (TEE), proteins C and S, antithrombin III, anti-phospholipid antibody, antinuclear antibody, perinucear anti neutrophilic cytoplasmic antibody, cytoplasmic anti-neutrophilic cytoplasmic antibody were all negative or inconclusive. Mild elevation of serum homocysteine was detected. Antinuclear antibody (ANA) was weakly positive but ANA blot was negative. Serial examinations during the acute stage of all stroke events revealed that the blood eosinophil counts gradually increased from 9% to 22% with mild leucocytosis. A high serum immunoglobulin E (IgE) level was also noted. No parasites or ova were found on stool examination. However after analysing all the reports it was noted that there was a progressive rise in eosinophil count and serum creatinine.
Tale of Previous Strokes
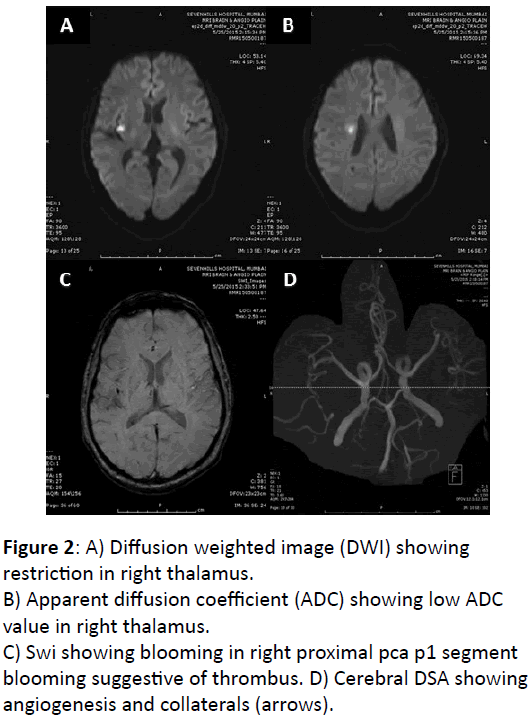
1st stroke 3 years ago patient developed mild dysarthria, facial weakness and confusion which resolved completely. MRI brain showed acute lacunar infarcts in right insular cortex and corona radiata with small thrombus in m2 segment. No significant stenosis on MR angiogram (Figure 2A-D). He was initiated on dual antiplatelets statins and he remained fine for a year
Figure 2: A) Diffusion weighted image (DWI) showing restriction in right thalamus.
B) Apparent diffusion coefficient (ADC) showing low ADC value in right thalamus.
C) Swi showing blooming in right proximal pca p1 segment blooming suggestive of thrombus.
D) Cerebral DSA showing angiogenesis and collaterals (arrows).
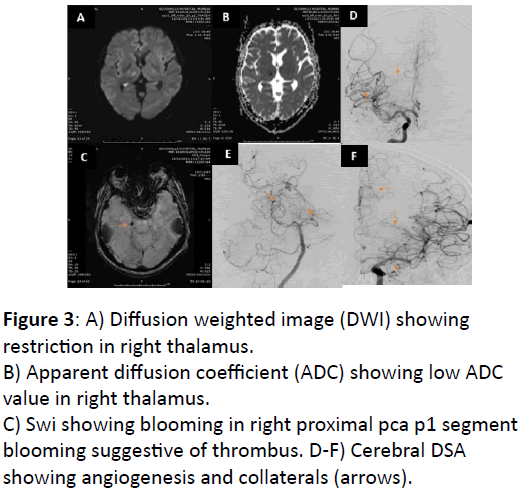
2nd stroke a year later developed left sided sensory complaints with MRI brain showing lacunar infarct in right thalamus and insular cortex. Small thrombus was seen in right pca distal p1 segment. Patient had stopped antiplatelets hence it was considered as non-compliance. Cerebral digital substraction angiogram (DSA) revealed multiple angiogenesis and collaterals in anterior and posterior circulation (Figure 3A-F).
Figure 3: A) Diffusion weighted image (DWI) showing restriction in right thalamus.
B) Apparent diffusion coefficient (ADC) showing low ADC value in right thalamus.
C) Swi showing blooming in right proximal pca p1 segment blooming suggestive of thrombus.
D-F) Cerebral DSA showing angiogenesis and collaterals (arrows).
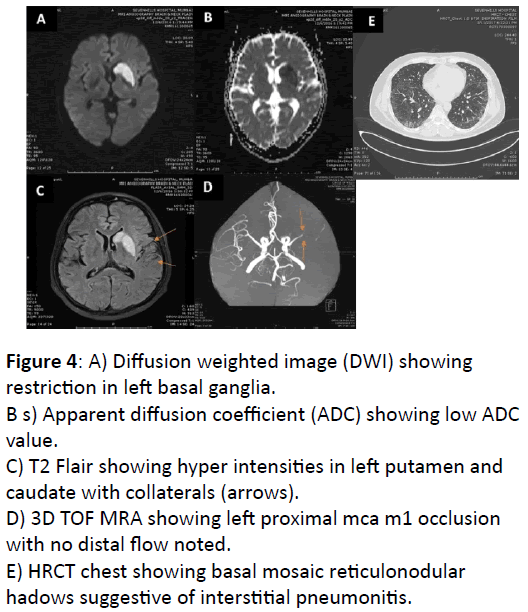
3rd stroke developed another year later when he had acute onset aphasia and brief right sided weakness. MRI brain revealed left basal ganglia infarction with left m1 occlusion with good collaterals (Figure 4A-E). He was initiated continued on dual antiplatelets and statins.
Figure 4: A) Diffusion weighted image (DWI) showing restriction in left basal ganglia.
B s) Apparent diffusion coefficient (ADC) showing low ADC value.
C) T2 Flair showing hyper intensities in left putamen and caudate with collaterals (arrows).
D) 3D TOF MRA showing left proximal mca m1 occlusion with no distal flow noted.
E) HRCT chest showing basal mosaic reticulonodular hadows suggestive of interstitial pneumonitis.
4th and final stroke despite best medical management, patient developed left sided weakness and dysarthria as described.
Multiple speciality consultation including Nephrology, cardiology, rheumatology and hematology consultations were obtained. Only positive finding was progressive rise in eosinophil count with worsening severity of stroke with worsening neurodeficit. Initially strokes were lacunar progressively they became large artery strokes.
After reviewing all investigations positive findings were patient had moderate eosinophilia with normal peripheral smear and bone marrow. Also genetic mutation for clonal eosinophilia was negative. Considering it as hypereosinophilic syndrome, patient was initiated on oral steroids. Absolute eosinophil count came down to 32 after initiating steroids. He continued oral prednisolone 60 mg per day for two weeks, and the eosinophil counts remained within the normal range. Tapering of steroid was successful, with no recurrence of eosinophilia on daily 10 mg prednisolone. The neurological deficits improved with reduction in creatinine levels. There have been no further episodes of stroke ever since starting steroids and controlling eosinophil counts.
Discussion
Studies have shown that about 30% strokes tend to recur and these recurrent strokes tend to be more disabling and fatal than the 1st stroke. A potential recurrent stroke is defined as any new acute neurological event with symptoms lasting more than 24 hours occurring after the initial ictus of the incident stroke that was not attributable to edema, brain shift, hemorrhagic transformation, intercurrent illness, hypoxia, or drug toxicity [1]. Various vascular risk factors like hypertension, dyslipidemia, coronary artery disease, cardiomyopathy, atrial fibrillation, atherosclerotic cerebrovascular disease and vasculitis are known to cause recurrent strokes [2]. One rare though important cause to look out for is Hypereosinophilia.
Eosinophilia could be due to primary or secondary cause. Commonest cause being parasite infection, atopy/allergic disorders, medications, toxins, autoimmune diseases, endocrine disorders etc. Primary eosinophilia includes clonal disorders like acute leukemia and chronic myeloid disorder and idiopathic eosinophilia [3]. In conditions where no exact cause can be determined is labelled as idiopathic eosinophilia. One unique subcategory of idiopathic eosinophilia being hypereosinophilic syndrome (HES).
Chusid et al. proposed diagnostic criteria for HES which consisted of: 1 Persistent eosinophilia >1,500 /μL for more than 6 months (or death before 6 months associated with signs and symptoms of hypereosinophilic disease). 2 Lack of evidence for parasitic, allergic or other known causes of eosinophilia. 3 Presumptive signs and symptoms of organ involvement, including hepatosplenomegaly, organic heart murmur, congestive heart failure, diffuse or focal nervous system abnormalities, pulmonary fibrosis, fever, weight loss and anemia.
Clinical manifestations of HES include multiple organ involvement like heart, skin, lung, nervous system and gastrointestinal tract. Patients often have non-specific symptoms like fatigue, myalgia, rash, low grade fever, cough and dysnoea [4]. Cardiac manifestation includes myocarditis, endocardial thrombi or endomyocardial fibrosis causing fatal restrictive cardiomyopathy [5]. Electrocardiogram may show non-specific and diffuse T-wave inversion. Echocardiogram and cardiac magnetic resonance imaging (MRI) are sensitive modalities for the detection of endomyocardial fibrosis and intracardiac thrombi [5]. Endomyocardial biopsy is the gold standard for diagnosis of endomyocardial fibrosis. Pulmonary symptoms may include dry cough, dyspnoea, and chest discomfort. Radiological findings are non-specific and may include infiltrates or diffuse interstitial pneumonia and fibrosis. Cutaneous manifestations include pruritis, erythematous rash, papules, nodules, eczema-like lesions, and urticaria-like eruptions. Mucosal ulcerations are more common in M-HES, whereas angioedema with elevated IgE levels is suggestive of L-HES variant. Vascular manifestations may be due to small vessel vasculitis or large vessel involvement causing digital necrosis, thrombophlebitis or Budd-Chiari syndrome. Our patient satisfied criteria for hypersoinophilic syndrome. He had fatigue, myalgia and dry cough. Absolute eosinophil count varied between 1500 to 3200 per cu.mm. HRCT chest suggestive of interstitial pneumonia like pattern
Neurological manifestations include recurrent stroke, encephalopathy and peripheral sensory neuropathies [5]. Although the exact pathogenesis is not well known, eosinophil accumulation appears to have pathological consequences. Eosinophils cause direct cytotoxiciy through the local release of toxic substances including cationic proteins, enzymes, reactive oxygen species, pro-inflammatory cytokines, and arachidonic acid derived factors [6,7]. The degree of end-organ damage is heterogeneous, and usually there is no correlation between the level or duration of eosinophilia and the severity of organ damage. Cardiac involvement is often the source of the thromboembolism, with thrombi formation in areas of damaged endocardium. Through the development of thromboemboli, patients with HES may experience embolic strokes or transient ischemic attacks, which may be multiple and recurrent. Initially strokes are small infarcts and borderzone. As the disease progresses, large artery strokes occur [5]. The second important clinical feature is encephalopathy presents as change in behavior, confusion, ataxia, and memory loss, and exhibit upper motor neuron signs with increased muscle tone, deep tendon reflexes, and a positive Babinski sign. Exact pathogenesis not known but it is directly related to absolute eosinophil count and cerebral infarcts [5]. Our case had recurrent strokes as described which to begin with small artery strokes but as the disease progressed and eosinophil count increased became large artery strokes. Also patient later developed mild confusion, psychomotor is slowing suggestive of encephalopathy due to recurrent strokes. Cardiac work up didn’t show any valve or endomyocardial lesion/vegetation.
In our patient, neuroimaging studies revealed multiple infarcts in the territories of both anterior and posterior circulations. However, no cardiac thrombus or structural anomaly was found transthoracic and transeosphageal echocardiogram. Cerebral DSA revealed neoangiogenesis with collaterals and irregularities in right PCA. Hence possible embolism from artery to artery was possible. Direct eosinophilic toxicity to a vascular wall, either on arterial or venous vessels, has been found in sparse reports.
Other clinical features include abdominal pain, diarrhoea, ascites, pancreatitis and cholangitis may be manifestations of gastrointestinal tract involvement. Hepatomegaly and splenomegaly are common in M-HES [6].
There is no permanent cure for HES and various therapies tried have been found to be inadequate. The goals for the treatment of HES are to reduce peripheral and tissue levels of eosinophils, and prevent end-organ damage and thromboembolic events in patients at risk. Antiplatelet agents and anticoagulants have no effect in thromboembolic prevention although in case with suspected cardioembolic strokes with hypereosiophilia it can be advocated [8]. Corticosteroids are considered the first line therapy [9]. Corticosteroids remain the first line treatment for most patients with HES, with the exception of PDGFRA-associated HES which is a type of HES where FIP1L1 indeed represent chronic eosinophilic leukemia, because these patients have a molecular genetic abnormality, peculiarly an FIP1L1–PDGFRA fusion gene.
The most appropriate initial dose and duration of treatment have not been studied but a dose of prednisone ≥ 40 mg has been suggested. Most patients respond to this dose. The dose should than be tapered very slowly to the lowest possible dose whilst monitoring the eosinophil count closely. Evaluation of bone density and adjunctive treatment to prevent bone loss is required in those requiring long-term steroid treatment. Highdose prednisolone is typically initiated at a dose of 1 mg/kg/day [10-13]. Once eosinophilia is properly controlled, the drug can be tapered gradually. Chronic maintenance therapy has been recommended as a more effective method to prevent the development of end-organ damage. Patients who will respond to corticosteroid therapy can usually be identified early in the course of therapy, often on the first day, as in our case. For patients refractory to steroids, the alternatives include cytotoxic agents (hydroxyurea, vincristine, cyclophosphamide), biological response modifiers (interferonalpha, cyclosporine), or targeted therapy (imatinib mesylate, mepolizumab) [14].
Conclusion
Recurrent strokes are a cause for major concern as with every stroke there is worsening of the neurological status. Apart from secondary prevention, most essential part of treatment is to find the etiology of recurrent stroke and treat it so as to prevent recurrence. Hypersoinophilia though a common entity, but if identified early and treated in case of stroke may prevent future recurrences and disability. For stroke patients without traditional vascular risk factors, an appropriate differential count of white blood cells would be indicated in order to detect this rare and treatable disease.
Conflict of Interest
The author(s) declare(s) that there is no conflict of interest regarding the publication of this paper.
References
- Coull Aj, Rothwell Pm (2004) Underestimation of the Early Risk of Recurrent Stroke Evidence of the Need for a Standard Definition. Stroke 35: 1925-1929.
- Leoo T, Lindgren A, Petersson J, Von Arbin M (2008) Risk Factors and Treatment at Recurrent Stroke Onset: Results from the Recurrent Stroke Quality and Epidemiology (RESQUE) Study. Cerebrovasc Dis 25: 254-260.
- Wei-Lun Chang, Huey-Juan Lin, He-Hsiung Chen (2008) Hypereosinophilic Syndrome with Recurrent Strokes: A Case Report. Acta Neurol Taiwan 17: 184-188.
- Weller PF, Bubley GJ (1994) The idiopathic hypereosinophilic syndrome. Blood 83: 2759-2779
- Khwaja GA, Duggal A, Kulkarni A, Choudhary N, Gupta M, et al. (2013) Hypereosinophilia–An Unusual Cause of Multiple Embolic Strokes and Multi-Organ Dysfunction. J Clin Diag Res 7: 2316-2318.
- Roufosse F, Cogan E, Goldman M (2003) The hypereosinophilic syndrome revisited. Annu Rev Med 54: 169-184.
- Gleich GJ (2000) Mechanisms of eosinophil-associated inflammation. J Allergy Clin Immunol 105: 651-663.
- Mankad R, Bonnichsen C, Mankad S (2016) Hypereosinophilic syndrome: cardiac diagnosis and management. Heart 102: 100-106.
- Gleich GJ, Leiferman KM (2009) The hypereosinophilic syndromes: current concepts and treatments. British J Haema 145: 271–285.
- Roufosse F, Bartholome E, Schandene L, Goldman M, Cogan E (1998) The idiopathic hypereosinophilic syndrome: clinical presentation, pathogenesis and therapeutic strategies. Drugs Today (Barc) 34: 361-373.
- Yoon TY, Ahn GB, Chang SH (2000) Complete remission of hypereosinophilic syndrome after interferon-alpha therapy: report of a case and literature review. J Dermatol 27: 110-115.
- Klion AD, Law MA, Noel P, Kim YJ, Haverty TP, et al. (2004) Safety and efficacy of the monoclonal anti-interleukin-5 antibody SCH55700 in the treatment of patients with hypereosinophilic syndrome. Blood 103: 2939-2941.
- Sefcick A, Sowter D, DasGupta E, Russell NH, Byrne JL (2004) Alemtuzumab therapy for refractory idiopathic hypereosinophilic syndrome. Br J Haematol 124: 558-559.
- Mulvey JJ, Magro C,Chadburn A (2017) Resolution of a steroid-resistant, hypereosinophilic immune diathesis with mepolizumab and concomitant amelioration of a mixed thrombotic microangiopathy. Blood Cells Mol Dis. pii: S1079-9796(17)30075-X.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences