Closed Loop System Design for Wireless Artificial Pancreas
Mohammed El Hadi, Ragda Mamoun, Omer Adam and Emtithal Ahmed
DOI10.21767/2471-299X.1000070
Mohammed El Hadi, Ragda Mamoun*, Omer Adam and Emtithal Ahmed
Department of Biomedical Engineering, University of Medical Sciences and Technology, Khartoum, Sudan
- *Corresponding Author:
- Ragda Mamoun BSc
Department of Biomedical Engineering, University of Medical Sciences and Technology, Khartoum, Sudan
Tel: +249 96 809 9106
E-mail: ragda.mamoun.8@gmail.com
Received date: June 15, 2018; Accepted date: July 11, 2018; Published date: July 20, 2018
Citation: Hadi ME, Mamoun R, Adam O, Ahmed E (2018) Closed Loop System Design for Wireless Artificial Pancreas. Med Clin Rev 4:7. doi: 10.21767/2471-299X.1000070
Copyright: © 2018 Hadi ME, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Over the years diabetes has been a major cause of death due to its severe complications. Therefore, the artificial pancreas was introduced to help type 1 diabetic patients to automatically control their blood glucose level noninvasively by providing the substitute endocrine functionality of a healthy pancreas. The output voltage of the pulse oximeter was related to glucose using Microsoft Excel Regression Analysis Tool. The glucose level is then transmitted wirelessly through the radio frequency zigbee module. Afterwards, it was regulated according to the treat to range control algorithm within the sliding scale insulin protocol.
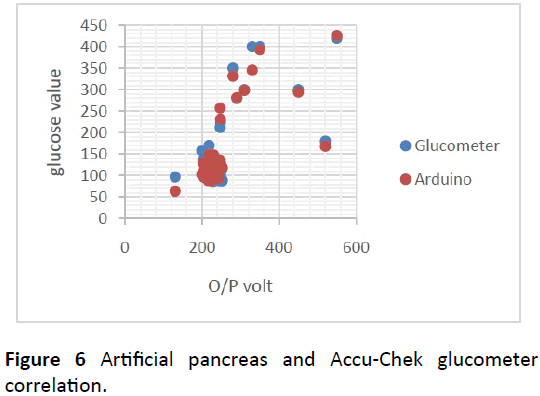
Although the artificial pancreas has managed to monitor blood glucose level and regulate it, its accuracy was found to be 89% compared to Accu-Chek glucometer. Not to mention its exceedingly inadequate size, time consumption and high expenses.
Keywords
Pancreas; Glucose; Insulin; Hypoglycemia; Hyperglycemia
Introduction
Diabetes from the Greek word meaning “a siphonÃÆâÃâââ¬Ãâà ¸ when the body is acting as a conduct for the excess fluid, and mellitus from the Greek and Latin for honey [1], which is a chronic disease that occurs when the pancreas is not producing enough insulin or when the body cannot effectively use the insulin it produces [2]. It is classified into four categories including: Type 1 which occurs when there is a Lack of insulin and requires insulin injections. While Type 2 occurs when there is insulin resistance (cells failure) and requires replacement. In addition, gestational diabetes occurs during pregnancy (might lead to type 2 diabetes). While pre-diabetes is when the glucose levels are higher than normal and lower than diabetic which eventually may lead to diabetes [3].
Type 1 diabetic patients must receive long acting basal insulin (Galrgine) before bedtime to take care of basal (steadystate) needs and rapid acting insulin (Lispro) before each main meal to compensate for meals or snacks. And the total daily dose (TDD) of the insulin must not exceed 0.5 units/kg, 40% of it is injected as long acting insulin while 60% is served as a rapid acting insulin divided into three meals a day (20% per meal) [4,5]. While Type 2 diabetes considered a milder form of diabetes because of its slow onset (pre-diabetes) and because it can be controlled with diet and medication [6].
The artificial pancreas (AP) is an externally worn medical device that mimics the endocrine function of the biological pancreas by monitoring blood glucose level (BGL). It infuses insulin and possibly glucagon according to an appropriate control law to regulate BGL to type 1 diabetic patient in a more automated fashion [7]. Offering mobility, reliability since it will automatically regulate and control glucose level by using digital communication techniques and advanced computer algorithms [8]. As well as avoiding hyperglycemia (raise in glucose levels) which over time contribute to complications development such as: nerve, kidney, eyes, muscle and foot damage, cardiovascular diseases and Alzheimer’s [9,10], while hypoglycemia (drop in glucose levels) which can be distressing and can lead to coma or death [7].
Fundamental Theories
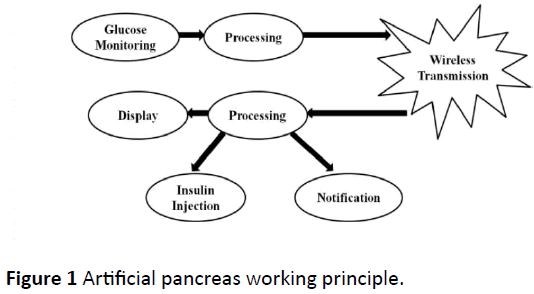
In the AP, the probe pulse oximeter’s output voltage is detected non-invasively and glucose is calculated within the first processing unit (arduino UNO). Then glucose is transmitted wirelessly through zigbee module to the second processing unit, displaying it in a liquid crystal display (LCD) for user interface to gain patient’s trust. According to the received glucose level and the treat to range control algorithm within the sliding scale insulin protocol the arduino UNO will inject insulin through an insulin pump composed of a DC motor (suction), insulin reservoir and infusion set during hyperglycemia, or do nothing if the glucose level is within the normal range. It will also notify the patient to eat something sweet in the case of hypoglycemia or get immediate help in the case of severe hyperglycemia by activating an alarm and locating the patient with a global positioning system (GPS) module (Figure 1).
Glucose calculations
Since the pulse oximeter probe is used as a non-invasive mean for monitoring glucose. Therefore, a relation between its analog to digital conversion (ADC) output voltage and glucose must be identified. The simplest way for understanding this relation is by deriving a mathematical equation that correlates both of them using regression [11]. As shown in the equation below:
Equation 1: Glucose-Voltage Relationship:
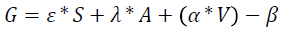
Where:
S: Sex of the patient “male=0 or female=1”.
A: Age of the Patient “years”.
G: Glucose concentration in the blood “mg/dl”.
V: ADC output voltage “volt”.
ÃÆââ¬Â°Ãâââ¬Âº , λ, α and β are constants, where ÃÆââ¬Â°Ãâââ¬Âº=19.71, =4.15, α=0.27 and β=54.16.
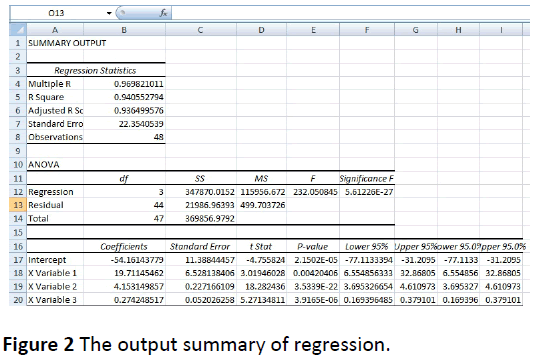
Equation 1 was done by monitoring glucose levels of random population using ACCU CHECK glucometer. By comparing its readings with pulse oximeter’s output voltage the equation was identified using Microsoft Excel regression analysis tool. And the analysis output is summarized in Figure 2.
Control algorithm
As seen in Table 1, the arduino will react with every specific level of glucose, following a treat to range (TTR) control algorithm based on the sliding scale insulin protocol [12].
| Blood Glucose Level, mg/dl | Insulin Endured, IU | Action Requested | |
|---|---|---|---|
| 0-80 | 0 | Alarm ON, GPS Activation | Hypos |
| 81-100 | 0 | No action | Normal |
| 101-150 | 0 | No action | T2DM |
| 151-200 | 2 | No action | |
| 201-250 | 4 | No action | |
| 251-300 | 6 | No action | T1DM |
| 301-350 | 8 | No action | |
| 351-400 | 10 | No action | |
| >401 | 12 | Alarm ON, GPS Activation | Hyper |
Table 1: Artificial pancreas control algorithm.
To convert IU (Insulin Unit) to mL divide by 100 to reduce 50 mg/dl [4].
Artificial pancreas construction
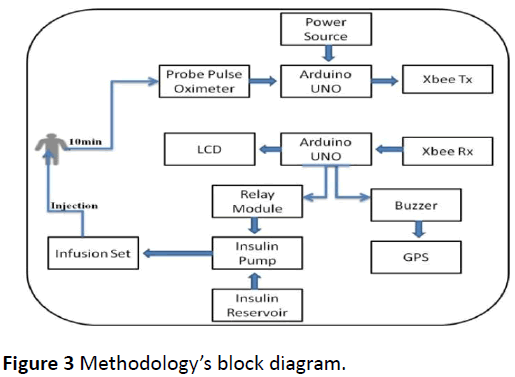
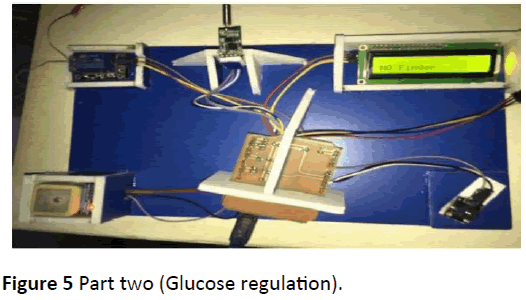
The AP (Figure 3) consists of two separate parts connected wirelessly using radio frequency zigbee module. The first circuit consists of a non-invasive glucose sensor (probe pulse oximeter), arduino UNO for control and zigbee transmitter. While the second part of the AP consist of zigbee receiver, arduino UNO, GPS, buzzer, 1-channel relay module, DC motor, insulin reservoir and infusion set (IV drip) (Figure 4).
The raw materials were collected and inspected, the Pulse Oximeter’s Probe analog to digital conversion (ADC) signal was sent to the arduino through the analog pin A0, while the arduino was programmed to send the signal wirelessly via zigbee transmitter connected to its digital pins (2 and 3) (Figure 5).
The GPS was connected to digital pins 10 and 11 in the arduino, while the DC motor was derived by the 1-Channel Relay connected to pin 6 in the arduino to suction insulin from the insulin reservoir and deliver it to the patient through an infusion set inserted subcutaneously. In addition to that, LCD pins were connected with pin A4 and A5 (analog) in the arduino based on the standard connection. Noticing that, the arduino was powered via its USB interface.
Results and Discussion
Results were categorized into two scenarios: the first scenario (Table 2) was precisely used to reveal how the artificial pancreas will react to all possible BGLs. The arduino in the second part was adjusted to estimate random glucose levels every 30 seconds (50 mg/dl-420 mg/dl) and react to it by either injecting insulin, activating the alarm and GPS or resulting in absolutely nothing. According to the treat to range control algorithm mentioned in Table 1 all the glucose ranges mentioned above will be displayed in the LCD. While in the second scenario shown in Table 3 the probe pulse oximeter glucose level was compared to the readings of Accu-Chek glucometer for error calculations. As a result, the output of the second part will interact accordingly to the first scenario.
| Item no | AP's Readings "mg/dl" | LCD Message | Action Taken |
|---|---|---|---|
| 1 | 50 | Range 1 | Buzer and GPS activation |
| 2 | 90 | Range 2 | No Action |
| 3 | 120 | Range 3 | No Action |
| 4 | 160 | Range 4 | Insulin Injection (2 ml) |
| 5 | 220 | Range 5 | Insulin Injection (4 ml) |
| 6 | 260 | Range 6 | Insulin Injection (6 ml) |
| 7 | 320 | Range 7 | Insulin Injection (8 ml) |
| 8 | 360 | Range 8 | Insulin Injection (10 ml) |
| 9 | 420 | Range 9 | Insulin Injection (12 ml), Buzer and GPS Activation |
Table 2: First scenario.
| Patient No | Gender | Age | O/P Voltage "V" | ap Readings "mg/dl" | Glucometer Readings "mg/dl" | Error "%" | Action Requested |
|---|---|---|---|---|---|---|---|
| 1 | Female | 21 | 207 | 108 | 105 | 3 | No Action |
| 2 | Male | 25 | 225 | 110 | 111 | 1 | No Action |
| 3 | Female | 22 | 213 | 114 | 119 | 4 | No Action |
| 4 | Male | 25 | 229 | 113 | 120 | 7 | No Action |
| 5 | Female | 21 | 202 | 107 | 108 | 1 | No Action |
| 6 | Male | 59 | 247 | 258 | 212 | 18 | Insulin Injection |
| 7 | Female | 21 | 212 | 110 | 114 | 4 | No Action |
| 8 | Male | 24 | 213 | 103 | 109 | 6 | No Action |
| 9 | Female | 48 | 248 | 231 | 224 | 3 | Insulin Injection |
| 10 | Female | 21 | 220 | 112 | 129 | 13 | No Action |
Table 3: Second scenario.
10 examples from a collection of 48 glucose samples are illustrated in Table 3 summarizing the second scenario:
As shown in Figure 6, the actual glucose levels computed using the artificial pancreas and the desired glucose levels conducted via the ACCU-CHEK glucometer were plotted together to clarify their correlation, due to voltage-glucose relation.
Conclusion
The artificial pancreas has managed to monitor the glucose level by calculating it from the probe pulse oximeter’s output voltage, using an equation derived with Microsoft Excel Linear Regression Technique. The glucose level was successfully regulated non-invasively, based on the signal received from the glucose meter wirelessly. As well as the control algorithm built on the treat to range sliding scale insulin protocol with an accuracy of 89%.
Recommendations
1. The AP should be patient specific because it is affected by various physiological features. Therefore, a physiological model including age, gender and weight must be considered to reduce the regression equation standard error as much as possible.
2. The smaller the device the more acceptability it will receive from patients, as it offers mobility. To minimize the size of the artificial pancreas microelectronics, integrated circuits (ICs) or nanotechnology can be used. If nanotechnology is used the system can simply be composed of a nano-glucose sensor working in cooperation with the biological pancreas, to emphasize its endocrine function activating it by transmitting electrical signals to control insulin production into the blood stream.
3. The artificial pancreas can be enhanced to endure multiple hormones not only insulin. It can also be used to deliver chemo/cryotherapy for pancreatic cancer patients since these surgeries cannot be done easily. The AP might also kill the cancer cells or reduce them without the need for high risk surgeries.
4. The AP system can be constructed as a glucose sensor in form of a ring consisting of a light emitting diode (LED) transmitter and photodiode to detect and measure glucose. While the insulin part “connected to the ring using wireless connection” may be constructed as a watch to display glucose level, calculate the exact dose of insulin and inject it directly to the vein behind the watch, putting into consideration that mobility issues must be controlled.
5. Follow up of diabetes should be accompanied with a backup storage to keep the weekly/monthly/annual readings. The daily glucose level graphs can be emerged to help health care professionals in statistics, diabetes researches and checking the clinical improvement per patient through their electronic medical record. There also should be a power backup and notification to the patient that his/her batteries need to be replaced as soon as possible.
Acknowledgment
Our deep gratitude goes to our families, Mr. Hussein Abdelazim, Dr. Mazin Sirry, Miss. Marwa Ibrahim , Miss. Alaa Sharaf El Deen, Miss. Sarah Abdallah, Mr. El Hadi Haroon, Eng. Mawada Siddig, Eng. Mohammed Abdullah, Eng. Ahmed Abdallah, Eng. Ayman Abbas, Eng. Fatima Noor El deen, Dr. Abd El Rahman Idris, Dr. Omer Homeida, Dr. Hassan Homeida, Dr. Hussein Homeida for their precious advices and efforts, not forgetting our lovely colleagues who had a huge contribution in our research including: Mujahid Ali, Amar Mithgal, Zainab Adil, Khawla Bashir, Ahmed Hatim, Esra Ali, Abubakr Muhammed, Walaa Hashim, Wafaa Suliman and Shahad Bakry.
References
- Bilous R, Donnelly R (2010) Handbook of diabetes. Wiley-Blackwell.
- Definition, diagnosis and classification of diabetes mellitus and its complications (1999) World Health Organization: Geneva.
- Ashok Khanna SD, Runkana V, Maheshwari S (2014) Personalized Artificial Pancreas Device System: India.
- Michal Ajzensztejn BW (2015) Guideline for the management of children and young people with newly diagnosed diabetes not in diabetic ketoacidosis (DKA). Evelina London Children’s Hospital.
- Bequette BW, Cameron F, Baysal N, Howsmon DP, Buckingham BA, et al. (2016) Algorithms for a Single Hormone Closed-Loop Artificial Pancreas: Challenges Pertinent to Chemical Process Operations and Control. Processes 4: 39.
- Kearns JMJ, Schork JS (2011) Artificial Pancreas. Diabetes Mine Design Challenge Winner.
- Hovorka DR (2017) Research Spotlight- The Artificial Pancreas. Diabetes UK.
- Artificial Pancreas, in An update from the Advanced Technologies & Treatments for Diabetes (ATTD) Conference (2013) N.H.S.R. Intelligence.
- Pepatheodorou K, Papanas N, Banach M, Papazoglou D, Edmonds M (2016) Complications of Diabetes. J Diabet Res 3.
- Kumar V, Cotran RS, Robbins SL (1997) The Pancreas, in Basic Pathology, J.M. Crawford, Editor, W.B. Saunders Company: USA 569.
- Rawlings JO, Pantula SG, Dickey DA (1998) Applied regression analysis: A research tool Springer USA, NEW YORK Berlin Heidelberg. 671.
- Okabayashi T, Nishimori I, Yamashita K, Sugimoto T, Maeda H, et al. (2009) Continuous Postoperative Blood Glucose Monitoring and Control by Artificial Pancreas in Patients Having Pancreatic Resection. JAMA NETWORK, Arch Surg 144: 933-937.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences