Effect of a Telephone Intervention upon Medication Adherence and Related Outcomes in Outpatients with Schizophrenia Spectrum Disorders (SSDs)
Lora Humphrey Beebe
DOI10.21767/2471-299X.1000048
Lora Humphrey Beebe*
University of Tennessee, Knoxville, Tennessee, USA
- *Corresponding Author:
- Lora Humphrey B, PhD
PMHNP-BC, University of Tennessee, 1200 Volunteer Blvd
Knoxville, TN 37996, USA
Tel: + 865 974 3978
E-mail: lbeebe1@utk.edu
Received date: May 27, 2017; Accepted date: June 13, 2017; Published date: June 20, 2017
Citation: Beebe LH (2017) Effect of a Telephone Intervention upon in Outpatients with Schizophrenia Spectrum Disorders (SSDs). Med Clin Rev 3:6. doi: 10.21767/2471-299X.1000048
Copyright: © 2017 Beebe LH. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Telephone intervention is an innovative option to provide needed problem solving interventions to improve medication adherence in Schizophrenia Spectrum Disorders (SSDs).
Methods: This randomized controlled trial examined the effect of weekly Telephone Intervention-Problem Solving (TIPS) upon quantitative and qualitative measures of psychiatric and nonpsychiatric medication adherence in 119 stable outpatients with SSDs for nine months.
Findings: Independent samples T-test revealed no significant differences in psychiatric or nonpsychiatric pill count adherence between groups; however 29% of experimental versus 19.6% of controls had serum antipsychotic levels within therapeutic range (NS).
Conclusion: This study extends the literature on adherence in SSDs by describing a clinical sample of stable outpatients for nine months. Our next project will examine the effectiveness of TIPS boosters upon outcomes.
Keywords
Schizophrenia; Outpatient; Adherence; Telemedicine
Introduction
Great number (upto 74%) of persons with schizophrenia spectrum disorders (schizophrenia, schizophreniform disorder and schizoaffective disorder-SSDs) are defined as nonadherent (take less than 80% of doses prescribed) to their antipsychotic medications [1], and poor adherence is leading contributor to SSD relapse [2]. Relapses increase disability, shorten remissions and reduce responsiveness to subsequent treatments, all of which increase the likelihood of costly rehospitalizations.
We conducted a randomized controlled trial to determine the effect of weekly telephone intervention upon subjective and objective measures of medication adherence in stable outpatients with SSDs for nine months.
Researchers and clinicians have grappled with the problem of SSD medication adherence for decades. Poor psychiatric medication adherence results in increased illness episodes associated with re-hospitalizations, longer time to remission and suicide attempts [2], all of which contribute to the exceedingly high personal and societal cost of SSDs. While the efficacy of antipsychotic medication for SSD maintenance is well documented [3], rates of nonadherence to antipsychotic medications in persons with SSDs range from 11-80% with average nonadherence exceeding 60% [4].
A primary problem in describing and intervening to promote SSD medication adherence, is defining and measuring the construct. While multiple measures of medication adherence are available, each is associated with a unique set of problems. Self-report is the most commonly used method of adherence monitoring, and the most likely to overestimate adherence [5]. Serum and urine testing is not available for all medications, and serum levels are known to be highly aversive [6]. A further limitation is that serum and urine levels provide adherence information only for the days immediately prior to testing [7]. Pharmacy refill rates are problematic if multiple pharmacies are used or if clients change pharmacies; further, pharmacy refill rates give no information about medication ingestion. Similarly, pill counts and electronic monitoring, while acceptable to clients, cannot be considered measures of actual medication ingestion [8].
Although an extensive body of literature suggests that in– person, problem-solving interventions can improve psychiatric medication adherence for persons with SSDs, such interventions are expensive and poorly suited for community implementation [9-11]. Where they even exist, problemsolving programs are offered to only the most severely ill persons due to cost and personnel constraints. This situation creates a need for effective, accessible and economical problem solving interventions for the majority of SSD outpatients. Using mobile telephones for intervention delivery might fill this need, but few studies have examined telephone intervention for SSDs.
We have conducted four prior studies of Telephone Intervention–Problem Solving for SSDs (TIPS), a manualized intervention in which trained nurses problem solve a variety of difficulties in community living that impact medication adherence and which has been described in detail elsewhere [12]. Our prior studies ranged from 6 weeks to 5 months length, with an average recruitment rate of 82.25% and an average retention rate of 84.75%. During the four studies, over 485 TIPS calls were provided. No participant experienced worsening symptoms as a result of TIPS, nor was any suicidal, homicidal, and psychiatric or other emergencies identified [13-16]. This prior work demonstrated the feasibility and acceptability of TIPS, and documented statistically significant improvement in psychiatric medication adherence over usual care for three months [15], but more information is needed on the relationships between objective adherence and subjective medication attitudes and self-efficacy in long-term, clinicbased samples of persons with SSDs.
This manuscript reports results from a randomized controlled trial we conducted to determine the effect of weekly TIPS upon in SSD outpatients over nine months.
The Theory of Planned Behavior (TPB) [17] guided this investigation. TPB [17] states that adherence intent is determined by individual attitude, subjective norm and perceived behavioral control (operationalized as self-efficacy) [17]. Attitudes reflect beliefs and values about perceived outcomes associated with adherence. Subjective norms reflect the extent to which significant others (family, caregivers) encourage adherence. Perceived behavioral control (selfefficacy) reflects the perceived ease or difficulty of overcoming adherence barriers and level of confidence in one’s ability to do so. TIPS addressed each of these determinants of adherence intent. During TIPS interventions, the nurse expressed and reinforced the value of adherence (subjective norm), educated participants about adherence benefits (attitude) and problem solved adherence barriers (perceived behavioral control/self-efficacy). In prior work, over half of SSD participants cited adherence barriers, most commonly memory and cognitive deficits [18]. Additional adherence barriers in prior work included attitudes toward illness, responses to undesirable medication side effects, lack of transportation, psychiatric symptoms, memory impairments, and substance use. TIPS provided a format for the provider to offer problem solving to mitigate the effects of these problems upon adherence.
Methods
We conducted a randomized controlled trial to determine the effect of weekly TIPS upon medication adherence in stable (not hospitalized in the past 6 months) outpatients with SSDs. Secondary analyses examined the relationships between subjective and objective adherence measures, medication attitudes and psychiatric symptoms.
Subjects were recruited from outpatients with SSDs receiving care at a Community Mental Health Center (CMHC) located in the southeastern United States. The CMHC is a regional, not-for-profit integrated system providing outpatient services to 650+adult (18 years and over) SSD outpatients. In addition to university Institutional Review Board (IRB) approval, signed letters of agreement and institutional consents were obtained before participants were recruited or data collected. HIPAA law and the Notice of Privacy Practices, signed by all patients at the CMHC, allows disclosure of Protected Health Information (PHI) for research, authorizing the initial case reviews and communications required to identify potential participants. After verifying this written authorization, we conducted record reviews to verify that participants met the following inclusion criteria: (a) A chart diagnosis of schizophrenia or schizoaffective disorder, any subtype, according to the criteria established in the Diagnostic and Statistical Manual of Mental Disorders [19], (b) Not hospitalized for psychiatric illness within the past 6 months, and (c) English speaking. Exclusion criteria were a chart diagnosis of coexisting mental retardation, neurological disorders or head injury, which could limit ability to complete study measures.
After verification of diagnoses via chart review, we met with potential participants in a private office at the CMHC to verify the remaining criteria. Following these verifications, we documented participants’ basic understanding of the study purposes and procedures using the Evaluation to Sign Consent (ESC) [20]. After a thorough explanation of the study, recruiters asked potential participants to answer 4 questions about the study. If all questions were answered correctly, written informed consent was obtained. If any question was answered incorrectly, study personnel repeated the information and asked the questions a second time. If any question was answered incorrectly the second time, study personnel waited at least 24 h before approaching the person again. After at least 24 h, the study was again explained and questions asked of the potential participant. If all questions were answered correctly, written informed consent was obtained. If any question was answered incorrectly during this second session, informed consent was not sought from that individual.
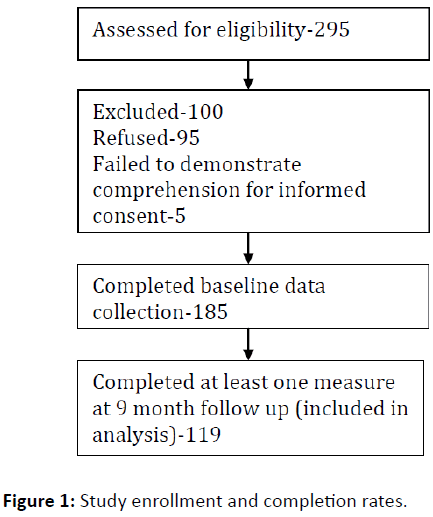
We approached a convenience sample of 295 potential participants (approximately 6 persons/week) over 13 months; to obtain baseline data on 185 persons with SSDs. Five persons were deemed ineligible due to failure to complete the ESC and 95 declined (Figure 1). Similar to our other investigations with this population [15,16], thirty three percent of eligible persons declined. Among those giving a reason, the most common reasons for declining were lack of interest (n=23, 24%) and being too busy (n=22, 23%). One hundred nineteen completed at least one measure at 9 month follow up for a retention rate of 64.3%.
Data regarding sociodemographic characteristics, living arrangements, educational level and prescribed medications was collected immediately following the granting of informed consent. Participants completed the Medication Adherence Rating Scale (MARS) [21] and the Medication Adherence Self- Efficacy Scale (MASES) [22]; trained study personnel completed the Positive and Negative Syndrome Scale (PANSS) [23] at study recruitment and again at nine months. Pill counts of all prescribed medications and serum levels of the primary antipsychotic medication were obtained within one month of study recruitment and repeated after nine months. Subjects were remunerated a $25 discount store gift card for the time required to complete the assessments at each measurement point.
Instruments
Medication Adherence Rating Scale (MARS)
The MARS [21] was used to measure self-reported medication adherence. The MARS was chosen because it has been previously used in persons with SSDs [21] and for brevity and ease of use. The scale contains 10 items measuring medication adherence behaviors, specific attitudes toward medication and the presence of negative side effects. Scores range from 1-10, with higher scores indicating better adherence. Cronbach’s Alpha for the MARS has been reported as 0.60-0.75. Test-retest reliability after 2 weeks was 0.72 [21]. Completion of the MARS took less than 10 min.
Medication Adherence Self-Efficacy Scale (MASES)
The MASES was used to measure self-reported confidence in one’s ability to adhere to prescribed medications (selfefficacy). The MASES [22] is a 26-item self-report scale that measures a person’s level of certainty (not at all sure=1; somewhat sure=2; or very sure=3) that they will be able to adhere to prescribed medications in a variety of common situations (for example, when busy at home, when in public, and when travelling). Items are summed and the mean calculated. Scores range from 1-3 with higher scores indicating higher medication adherence self-efficacy. Cronbach’s alpha for the MASES was 0.95 and one-week test retest reliability was 0.4. [22]. The MASES was designed to measure medication adherence self-efficacy in hypertensive African Americans and to our knowledge this is the measure’s first use in SSDs. We chose the measure for several reasons. Both hypertension and SSDs are chronic illnesses that necessitate daily medication management to prevent disability and illness exacerbations. Further, there exists no medication adherence self-efficacy measure specifically designed for persons with SSDs. Completion of the MASES took less than 10 min. To our knowledge, this is the first study to measure medication adherence self-efficacy in persons with SSDs.
Positive and Negative Syndrome Scale (PANSS)
The PANSS [23] was completed by trained study personnel at baseline and after three months. The PANSS is a 30-item Likert-type scale that measures schizophrenia symptoms. Scores range from 30-210 with higher scores indicating higher symptom levels. Cronbach’s a was 0.73 on the positive subscale, 0.83 on the negative subscale, and 0.87 on the general subscale. Internal reliability coefficients ranged from 0.70-0.85. Before administering the PANSS, all study personnel participated in online training from the developers. After a conference call to review the interview scales, definitions and scoring instructions, study personnel practiced scoring the PANSS using training tapes provided by the scale developers. Training continued until all raters achieved scores within +/- one point of the gold standard PANSS rating panel for all items. Cronbach’s Alpha for study personnel was 0.89. Completion of the PANSS took approximately 40 min.
Pill count a blinded Research Assistant (RA) performed pill counts in participant homes using an identical procedure regardless of group assignment. The RA initiated telephone contact to schedule a day and time for pill count. If no contact was made or if the participant was not home at the scheduled time, the pill count was be rescheduled within 1 week. All inhome pill count visits were conducted during daylight hours and attended by two research staff. Participants were asked to produce all oral prescription medications to be counted. For purposes of this project and based upon the medications most commonly prescribed to participants in prior studies, we defined psychiatric medications as: risperidone, haloperidol, clozapine, fluphenazine, olanzapine, aripiprazole, quetiapine, and ziprasidone. All others we defined as nonpsychiatric medications. A measure of adherence was generated by dividing the number of pills missing from the bottle(s) by the number of pills prescribed within the time period covered by the current prescription. Adherence percentages were calculated separately for each oral medication according to the formula:
Number of pills missing from the bottle/Number of pills prescribed during the current time period × 100
Adherence to depot medication was calculated by record review. The percent of injections documented out of the total injections ordered during the 9-month study period constituted the depot adherence percentage. If multiple medications were prescribed (psychiatric or nonpsychiatric), overall adherence was calculated by averaging the percent adherence of all medications within that category.
Serum medication levels
Serum antipsychotic medication levels were collected at the recruitment site at baseline and nine months regardless of group assignment. Laboratory personnel collected approximately 15 cc of blood from the antecubital space, and specimens were analyzed via liquid chromatography tandem mass spectrometry. Transportation (bus pass) was provided if necessary.
Findings
Data analysis began with data plots and basic descriptive statistics, such as frequency distributions, means and standard deviations, appropriate for the level of measurement of the variables. SPSS (19.0) was used to set up, enter and analyze the data.
Table 1 summarizes sample characteristics. The 119 outpatients completing baseline and at least one nine month measure ranged in age from 19-75 years with an average age of 47.6 years (SD 12.7). The sample was evenly divided along gender lines, however a majority of participants were diagnosed with schizoaffective disorder (n=77, 64.7%). Most were Caucasian (n=80, 67.2%); the remainder were African American (n=37, 31.1%) and Asian (n=2, 1.7%). Most participants were living with family when study measures were completed (n=64, 53.8%). The remainder lived alone (n=48, 40.3%) or with paid caregivers (n=7, 5.9%). Self-reported educational levels (note: missing data) were: graduated high school (42%), did not graduate high school (27%) and completed some post high school education (31%). The following characteristics were not significantly associated with serum medication level of antipsychotic medication at baseline: age, race, diagnosis, gender, living arrangement, educational level, or being prescribed depot antipsychotic medication. The following characteristics were not significantly associated with pill count adherence to psychiatric or nonpsychiatric medication at baseline: Race, diagnosis, gender, living arrangement, and educational level. Age was significantly negatively correlated with baseline pill count adherence, but only for non-psychiatric medications (r=-0.275, p=0.005). Baseline adherence (by pill count) to all psychiatric medications was significantly higher if the primary antipsychotic medication was administered by the depot route (t=2.4, df 99, p=0.02).
| Characteristic | n(%) |
|---|---|
| Diagnosis | |
| Schizoaffective | 77(64.7) |
| Schizophrenia | 42(35.3) |
| Sex | |
| Male | 59(49.6) |
| Female | 60(50.4) |
| Race | |
| Caucasian | 80(67.2) |
| African American | 37 (31.1) |
| Asian | 2(1.7) |
| Living Arrangement | |
| With family | 64(53.8) |
| Alone | 48(40.3) |
| With Caregiver | 7 (5.9) |
| Self-reported Educational Level* | |
| Less than High School | 33(27.7) |
| High School Graduate | 50(42) |
| More than High School | 31(30.3) |
| Prescribed Medications | |
| Oral atypicals | 83(69.7) |
| Oral typicals | 20(16.8) |
| Depot typicals | 20(16.8) |
| Depot atypicals | 15(12.6) |
| Antidepressants | 62(52.1) |
| Mood stabilizers | 40(33.6) |
| Antianxiety | 38(31.9) |
| Anticholinergics | 53(44.5) |
| Hypnotics | 28(23.5) |
| Other+ | 83(69.7) |
Table 1: Characteristics of persons with SSDs (N=119).
Psychiatric medications
T tests were conducted to examine differences in scores on the pill count and serum medication levels between groups at nine months. Baseline pill count psychiatric medication adherence averaged 63.7% (SD 28.5) for experimental participants and 70.1% (SD 19.95) for controls. Nine month pill count psychiatric medication adherence averaged 62.5% (SD 35.8) for experimental participants and 54.9 (SD 36.7) for controls (NS).
These pill count percentages represent the average adherence of all psychiatric medications prescribed, i.e., typical and atypical antipsychotics, antidepressants, antianxiety agents, mood stabilizers, anti-parkinsonian agents and hypnotics. Independent samples T test revealed no significant differences between groups at baseline, nor at the nine month measurement point. Finally, paired sample T tests revealed no significant change in mean psychiatric medication adherence from baseline to six months in either group.
Our findings on serum levels of the primary antipsychotic medication prescribed enhance our pill count data by focusing only on prescribed antipsychotics, i.e., risperidone, haloperidol, clozapine, fluphenazine, olanzapine, aripiprazole, quetiapine, and ziprasidone. Sixty six percent of experimental participants versus 50% of controls had serum antipsychotic levels within therapeutic range at baseline (NS). At the nine month measurement point, 29% of experimental versus 19.6% of controls had serum antipsychotic levels within therapeutic range (NS).
Nonpsychiatric medications
Experimental participants had significantly greater pill count adherence to nonpsychiatric medications at baseline (t=2.3, df 85, p=0.029) than controls. Baseline pill count adherence for nonpsychiatric medications averaged 67.4% (SD 30.2) for experimental and 52.2% (SD 32.2) for controls. Nine month pill count adherence for nonpsychiatric medications averaged 60.1% (SD 37.2) for experimental and 53.3% for controls (SD 34.9). Independent samples T test revealed no significant differences between the groups at nine months. Finally, paired sample T tests revealed no significant change in mean nonpsychiatric medication adherence from baseline to nine months in either group.
Other outcomes
T tests were conducted to examine differences in scores on the PANSS, MARS and MASES between groups at nine months. Symptoms were lower, self-reported medication adherence and medication adherence self-efficacy was higher in experimental participant after nine months, but differences were not statistically significant.
The Medication Adherence Rating Scale (MARS) scores averaged 7.2 (SD 1.9) for experimentals and 6.9 (SD 1.9) for controls at baseline (NS). MARS scores averaged 8.0 (SD 1.8) for experimentals and 7.5 (SD 2.2) for controls after 9 months (NS).
The Medication Adherence Self-Efficacy Scale (MASES) scores averaged 2.5 (SD 0.43) for experimentals and 2.4 (SD 0.42) for controls at baseline (NS). MASES scores averaged 2.53(SD 0.47) for experimentals and 2.38 (SD 0.59) for controls after 9 months (NS).
The Positive and Negative Syndrome Scale (PANSS) scores averaged 79.7 (SD 9.6) for experimentals and 79.4 (SD 8.8) for controls at baseline (NS). PANSS scores averaged 75.1(SD 9.8) for experimentals and 76.4 (SD 15.9) for controls after 9 months (NS).
The work reported here is our fifth study of TIPS. Prior studies ranged from 6 weeks to 5 months length, with an average recruitment rate of 82.25% and an average retention rate of 84.75% [14-16,18]. In this study, both recruitment and retention were lower than prior work-our recruitment rate was 68% (95 refused of 295 eligible) and retention was 53.5% (185 participants completed baseline measures, and 119 participants completed at least one measure at 9-months). Possible explanations include differing lengths of follow up, the inclusion of persons with schizoaffective disorder in the current investigation, and the use of serological measures that are known to be aversive and likely had a negative effect on both participation and completion rates [6].
Our pill count data indicates that participants in this investigation missed an average of 37.2% of psychiatric and 41.75% of nonpsychiatric medication doses during this nine month study. These adherence percentages are lower than the average nonadherence rate of 60% reported by others [2,4]. Compared to our prior work [15], average pill count psychiatric medication adherence in this study was lower for both experimentals and controls; average nonpsychiatric medication adherence in this study was higher for both groups. Differences may be due to differences in the samples examined: our prior work included only persons with schizophrenia while the current sample included a majority of persons with schizoaffective disorder. It is likely that the mood component associated with schizoaffective disorder is associated with polypharmacy, increasing complexity of medication regimen and possibly contributing to nonadherence. The longer (nine month) follow up period in this study likely impacted adherence rates as well, the Clinical Antipsychotic Trials for Intervention Effectiveness (CATIE) trial found that cessation of medication increased as length of follow up increased [24].
The current findings contrast somewhat with our prior work. Our three month follow up study documented statistically significant differences in pill count psychiatric medication adherence over three months in persons receiving TIPS compared to usual care [15]. Although findings were in the predicted direction, the current study found no statistically significant differences in pill count adherence over nine months, possibly due to the longer follow up period which may have attenuated the effects of the TIPS intervention over time. Further, longer follow up periods are associated with greater study attrition, which we also experienced compared to prior work. This situation introduces the possibility of nonidentification of significant effects based upon a decreased sample size over time. Our inclusion criteria in this current study, of community stability may have resulted in a more adherent sample as well, with the high rates of baseline adherence making it difficult to demonstrate statistically significant increases.
The study reported here is our first investigation to examine serum antipsychotic medication levels, and the serological findings illuminate and extend our pill count data. While the pill count data (measuring adherence for all psychiatric medications) did not demonstrate statistically significant differences, the serological data (measuring adherence only to antipsychotic medications) indicated that 62.7% of TIPS participants had antipsychotic levels within therapeutic range versus only 47.3% of controls at nine months-however this result was not statistically significant. This finding highlights the importance of detail and specificity in adherence investigations, and the necessity of including multiple measures of adherence to paint the most detailed picture possible of this complex behavior.
Our results must be interpreted in light of project limitations. Our use of a single study site limits generalizability, and our 46.5% attrition rate may have affected outcomes of interest. SSD adherence research is subject to selection bias, as non-adherent persons may be less likely to participate or complete study measures. Such bias is a limitation to both internal validity and external generalizability. Finally, our inclusion criteria of community stability means our results cannot be applied to recently hospitalized persons.
Conclusion
This study extends the literature on adherence in SSDs by describing a clinical sample of stable outpatients, extending the follow up period compared to our prior investigations, and examining adherence to both psychiatric and nonpsychiatric medications. Our next project will examine the effectiveness of TIPS boosters upon outcomes over time.
References
- Yang J, Ko YH, Paik JW, Lee MS, Han C, et al. (2012) Symptom severity and attitudes toward medication: impacts on adherence in outpatients with schizophrenia. Schiz Res 134: 226-231.
- Hegedus A, Kozel B (2014) Does adherence therapy improve medication adherence among patients with schizophrenia? A systematic review.Int JMH Nurs 23: 490-497.
- Leucht S, Tardy M, Komossa K, Heres S, Kissling W, et al. (2012) Antipsychotic drugs versus placebo for relapse prevention in schizophrenia: A systematic review and meta-analysis.Lancet 379: 2063-2071.
- Czobor P, Van Dorn RA, Citrome L, Kahn RS, Fleischhacker WW, et al. (2015) Treatment adherence in schizophrenia: A patient-level meta-analysis of combined CATIE and EUFEST studies.EurNeurPsychopharm25: 1158-1168.
- Ascher-Svanum H, Zhu B, Faries D, Lacro JP, Dolder CR (2006) A prospective study of risk factors for nonadherence with antipsychotic medication in the treatment of schizophrenia. J Clin Psychiatr 67: 1114-1123.
- Gilchrist PT, Ditto B (2010) The effects of blood-draw and injection stimuli on the vasovagal response. Psychophys 49: 815-821.
- Frangou S, Sachpazidis BA, Stassinakis A, Sakas G (2005) Telemonitoring of medication adherence in patients with schizophrenia. Tele Med E-health 11: 675-683.
- Nakonezny PA, Byerly MJ (2005) Electronically monitored adherence in outpatients with schizophrenia or schizoaffective disorder: A comparison of first vs second generation antipsychotics.Schiz Res 82: 107-114.
- Falloon IR, Held T, Roncone R, Coverdale JH, Laidlaw TM (1998) Optimal treatment strategies to enhance recovery from schizophrenia. Aust NZJ Psychiatr 32: 43-49.
- Liberman RP, Eckman TA, Marder SR (2001) Rehab rounds: Training in social problem solving among persons with schizophrenia. Psychiatr Serv 52: 31-33.
- Zygmunt A, Olfson M, Boyer CA, Mechanic D (2002) Interventions to improve medication adherence in schizophrenia. Amer J Psychiatr 159: 1653-1664.
- Beebe LH (2005) Telephone Intervention Problem Solving (TIPS) for Persons with Schizophrenia.DirPsychiatrNurs 2: 103-111.
- Beebe LH (2001) Community nursing support for clients with schizophrenia. Arch PsychiatrNurs 15: 214-222.
- Beebe LH, Tian L (2004) TIPS: Telephone Intervention-Problem Solving for persons with Schizophrenia. IssMentHlthNurs 25: 317-329.
- Beebe LH, Smith K, Crye C, Addonizio C, Strunk DJ, et al. (2008) Telenursing intervention increases psychiatric medication adherence in schizophrenia outpatients.J AmerPsychiatrNursAssoc 14: 217-224.
- Beebe LH, Smith K, Bennett C, Bentley K, Walters A, et al. (2010) Keeping in Touch: Cell Phone use in People with Schizophrenia Spectrum Disorders.J Psycho socNurs MH Serv 48: 32-37.
- Ajzen I (1991) The theory of planned behavior. Organic Behavior and Human Decisional Processes 50: 179-211
- Beebe LH (2002) Problems in community living identified by people with schizophrenia. J Psycho socNurs MH Serv 40: 38-46.
- American Psychiatric Association (APA) (2000) Diagnostic and statistical manual of mental disorders (4th edn.). Washington, DC: APA.
- De-Renzo EG, Conley RR,Love R (1998) Assessment of capacity to give consent to research participation: state-of-the-art and beyond. J HlthCare Law Pol 1: 66-87.
- Thompson K, Kulkari J, Sergejew AA (2000) Reliability and validity of a new Medication Adherence Rating Scale (MARS) for the psychoses. Schiz Res 42: 241 247.
- Ogedegbe G, Mancuso CA, Allegrante JP, Charlson ME (2003) Development and evaluation of a medication adherence self-efficacy scale in hypertensive African-American patients. J Clin Epidem 56: 520-529.
- Kay SR, Fiszbein A, Opler LA (1987) The positive and negative syndrome scale (PANSS) for schizophrenia. Schiz Bull 13: 261-276.
- Manschreck TC, Boshes RA (2007) The CATIE Schizophrenia trial: Results, Impact, Controversy. Harv Rev Psychiatr 15: 245-258.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences