Breast Cancer Detection and Screening
Elroy Patrick Weledji and Joshua Tambe
DOI10.21767/2471-299X.1000071
Elroy Patrick Weledji1* and Joshua Tambe2
1Department of Surgery, Faculty of Health Sciences, University of Buea, Cameroon
2Department of Radiology, Faculty of Health Sciences, University of Buea, Cameroon
- *Corresponding Author:
- Elroy Patrick Weledji
Department of Surgery, Faculty of Health Sciences
University of Buea, Cameroon
Tel: +237699922144
E-mail: elroypat@yahoo.co.uk
Received date: June 29, 2018; Accepted date: July 19, 2018; Published date: July 27, 2018
Citation: Weledji EP, Tambe J (2018) Breast Cancer Detection and Screening. Med Clin Rev 4:8. doi: 10.21767/2471-299X.1000071.
Copyright: © 2018 Weledji EP, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
The outcome of breast cancer is biologically predetermined by the presence or absence of micrometastases. The investigation of any breast abnormality must involve ‘triple assessment’-clinical examination, imaging and cytology. Each of these diagnostic modalities complements the others, and together improves the diagnostic yield. Screening for breast cancer in women aged 50-65 years enables the detection and treatment of cancers at an asymptomatic stage and the mortality can be reduced by a quarter. However, the problems of bias (lead time, length and selection) and interval cancers are the short-comings. In addition, there is an ongoing debate as to the value of mammographic screening in women under 50. The article reviewed the role of ‘triple assessment’ in the detection of breast cancer and the rationale for a breast screening programme.
Keywords
Breast cancer; Triple assessment; Screening
Introduction
Breast cancer is a public health problem as it is the second commonest cancer with increasing incidence (1 in 8 women aged 45-55) in the world. It is the second most common cause of death after lung cancer in the West [1]. It is important to note that although over 90% of breast disease is benign, breast cancer is easily diagnosed as the suspicious lump is mostly discovered by the patient who calls the attention of the physician. Most breast cancers are associated with fibrous tissue proliferation (scirrhous) and consequently the tissues surrounding the growth contract clinically and presents as dimpling of the skin and in-drawing of the nipple [2,3]. Local spread occurs in 40% of breast cancer patients at presentation and 75% of lymphatic drainage is to the ipsilateral axilla [4]. Following the definitive diagnosis of breast cancer using ‘triple’ assessment (clinical, pathological and radiological) the patient should have appropriate staging tests i.e. a metastatic work-up prior to decision on management [2,5]. The hypothesis underlying the screening for malignant disease is that the detection and treatment of cancers at an asymptomatic stage enables the cure of lesions which would be incurable if left until patients present with symptoms [6,7]. The paper reviewed the role of ‘triple assessment’ in the detection of breast cancer and the rationale for a breast screening programme.
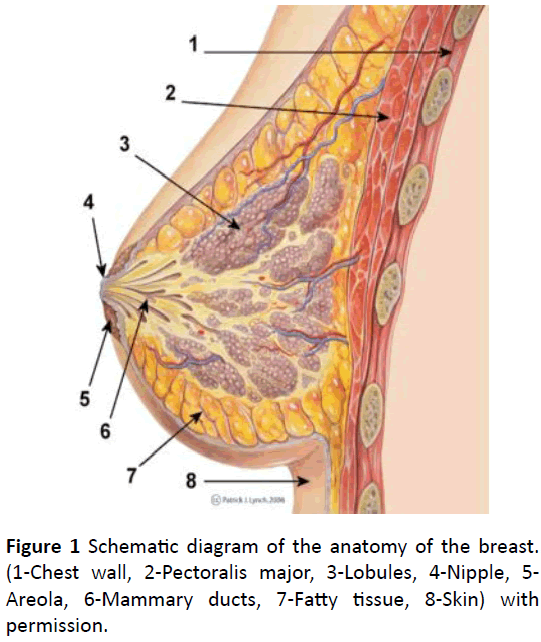
Breast anatomy
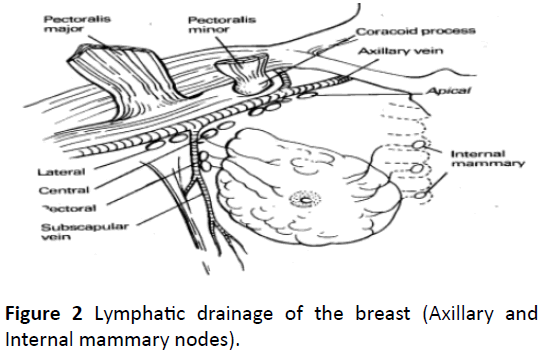
The female breast is a glandular (apocrine) organ, roughly hemispherical in shape, with a pigmented areola at its apex from which the nipple arises centrally. Within the breast, the glandular tissue is imbedded in fat and divided into approximately 15-20 lobes, each draining into a duct which reaches the skin at the nipple (Figure 1). The breast is situated in the superficial fascia of the anterior thoracic wall, and despite large variations in the dimensions of the breast, its base has a fairly constant size; overlying the second to sixth ribs, and extending from the lateral border of the sternum to the mid-axillary line. The axillary tail extends upward and lateral into the axilla, and is closely related to the nerve supply of latissimus dorsi (the thoracodorsal nerve) and serratus anterior (the long thoracic nerve of Bell). The arterial supply is derived from perforating branches of the internal mammary and intercostal arteries, with large vessels arising from the second and third intercostal spaces, and with an additional supply from the lateral thoracic artery. Lymphatic drainage is via subareolar and submammary plexuses to nodes along the internal mammary chain medially, and to the pectoral axillary nodes laterally, which drain via the central and apical groups to the supraclavicular and cervical nodes. 75% of the lymphatic drainage from the breast is to the ipsilateral axilla. The superior part of the breast drains to the infraclavicular and supraclavicular nodes and thence to the deep cervical node (Figure 2). Inferiorly, lymphatic drainage is through the abdominal wall and diaphragm to mediastinal nodes. There is free lymphatic communication across the midline between the two breasts. The male breast is rudimentary, comprising small ducts without alveoli and supported by fibrous tissue and fat. Insignificant it may be, but it is still prone to the major diseases that affect the female organ albeit not as common.
Breast cancer types
The WHO classifies breast cancer into (a) Epithelial which can be non-invasive ductal carcinoma in-situ (DCIS), lobular carcinoma in-situ (LCIS) or invasive ductal (85%), lobular (1%), mucinous (5%), papillary (<5%), medullary (<5%), (b) Mixed connective tissue and epithelial (c) Miscellaneous. The majority of breast cancers are adenocarcinomas arising from the epithelium of the ducts and lobules (ductal and lobular types) [2,3]. The histological types of invasive breast cancer are summarized in Table 1. Ductal with productive fibrosis is the commonest form. Lobular carcinomas have a high propensity for bilaterality, multicentricity and multifocality and have a particular propensity for metastasising to membrane structures, such as the peritoneum, pleura and meninges.
| Type | Frequency (%) |
|---|---|
| Ductal | 80 (non-specific 50%) |
| Lobular/ductal combined | 5 |
| Medullary | 6 |
| Colloid | 2 |
| Other less common specific types (tubular, papillary) | 2 |
| Sarcoma and lymphoma | 0.5 |
Table 1: Relative frequency of histological types of breast cancer.
Carcinoma in situ: Ductal carcinoma in situ (DCIS) is subdivided into commode, solid, cribiform and macropapillary patterns. Commode DCIS is associated with micro-invasive foci and lymph node metastases. Because necrosis and microcalcification are common and seen on mammography, DCIS is detected increasingly commonly. LCIS has no microcalcification and therefore may not be detected early. 10-30% of patients with LCIS and 30%-50% of those with DCIS go on to develop invasive cancer. LCIS may occur in either breast and is a marker of increased risk of diffuse bilateral disease, compared with DCIS, which remains in the ipsilateral breast and is confined to the same quadrant from which the biopsy that yielded the diagnosis was taken.
The ‘Triple Assessment’
The investigation of any breast abnormality must involve ‘triple assessment’-clinical examination, imaging and cytology. Each of these diagnostic modalities complements the others, and together improves the diagnostic yield [8,9]. None should thus be interpreted in isolation [10]. Women given the diagnosis of breast cancer by the surgeon should have an opportunity to talk with the breast care nurse about their disease and the options for treatment following a multidisciplinary team meeting. The patient is then able to participate in the choice of treatment.
Clinical assessment
History: Patients are referred to the specialist breast clinic for a variety of reasons (Table 2) [11]. The most common symptom is the belief by the patient that she has a lump in the breast, followed by breast pain. Breast pain without the presence of a palpable lump is not a symptom of breast cancer and is a common benign problem.
| Symptoms | Consecutive cases |
|---|---|
| Lump in breast | 933 |
| Tender ‘lumpy’ area | 110 |
| Breast pain | 224 |
| Nipple discharge | 75 |
| Nipple retraction | 36 |
| Nipple eczema | 3 |
| Swelling of breast | 7 |
| Others | 57 |
Table 2: The symptoms of 1445 consecutive cases referred to a specialized breast clinic [11].
Examination: The overwhelming majority of true lumps are cysts, fibroadenomas or carcinomas. Breast carcinoma is a discrete, irregularly shaped firm and immobile, painless lump attached to adjacent breast tissue. Both breasts and nodal drainage areas (axilla and supraclavicular fossa) should be carefully examined by visual inspection and palpation. The skill and experience in examination of the breast are essential. Under good lighting, on inspection the physical signs to look for are lump, inflammation and tether. The examiner should stand directly in front of the patient who sits on the edge of the examination couch. A lump may be clearly seen and inflammation of the nipple may manifest in a considerable area of excoriation of the nipple and areolar caused by Paget’s disease of the nipple. Skin tether from the fibrotic in-pulling of the tumour, and tether of the nipple (nipple retraction) is easily seen but, skin tether may be a much more subtle clinical sign, sometimes seen only when the patient raises her arms above her head.
Thus, the breasts are examined with the arms by the side and then the patient slowly raises her arms and places them behind the head. For palpation, the patient is propped up to around 30 degrees. In this position the breast is easiest to examine. If the patient is lying flat, the skin over the breast becomes more tense and the breast tissue is not as easy to palpate. The patient should be examined first with her arms by her sides, then with her arms placed on her head, and finally sitting up and leaning forward a little. If the lump cannot be felt, the patient is asked to put one finger on it. This a useful guide as if she goes straight to it with one finger then there is a true lump. If she feels vaguely around or picks up the breast tissue between her fingers, then there is no true lump. Palpation is with the flat of the fingers and the examiner should not press too hard nor pick up the tissue between the fingers. Some examiners prefer to make circular movement with the fingers as they feel the breast. Examination should include all the quadrants of the breast, the centre behind the nipple and the axillary tail. The breast deep to the nipple is palpated for an underlying lump and also if there is an associated nipple discharge. A palpated retracted nipple will not evert. It is important to decide which of the following circumstances apply: 1) a true lump or other abnormality such as nipple eczema is present. This requires a final diagnosis, the need of which is not changed by imaging, 2) the breasts are normal, 3) there is no definite abnormality but the examiner remains a little uncertain about, for example, a lumpy area with no true lump. Reassessment, either by imaging or by reexamination at a different time of the menstrual cycle, is advised. The characteristics of a malignant lump are noted as (1) a discrete, (2) irregularly shaped, (3) irregularly surfaced, (4) poorly defined edge, (5) hard consistency, (6) fixed to adjacent breast tissue, (7) with attachments to skin (tether/fixation) or to pectoralis fascia [3,11,12].
Axilla and supraclavicular fossa: Clinical examination for detecting nodes containing metastatic tumour is inaccurate with a sensitivity and specificity of less than 70%, i.e. false negatives and positives in up to a third [3,13,14]. Preoperative imaging is a little better with ultrasound having a sensitivity of only 73% [3]. The classifications on clinical examination are as follows: no palpable axillary nodes (N0); mobile axillary nodes (N1); fixed axillary nodes (N2); palpable supraclavicular nodes (N3/M1). Lymphoedema of the arm can be palpated. The abdomen is palpated for hepatomegaly as evidence of distant metastases (M1) [12].
Auscultation: The chest can be auscultated for pleural effusion or consolidation as evidence for distant metastases (M1).
Clinical staging (TNM) of breast cancer
Following clinical diagnosis and assessment of the lump and the patient for signs of metastatic spread a simple attempt is made at clinically staging the tumour according to the tumour (size), axillary node (mobility, fixity), metastases (distant metastases) (TNM) system [15]. The TNM staging system is commonly used as it gives more precise information about the extent of the cancer clinically [3]. However, the nodal status and size frequently change when the pathological measurements are given and there is thus little conformity as to which groups of patients are being compared. This is a short-coming of clinical staging [16]. The outcome of treatment of early breast cancer is biologically pre-determined by the extent of micrometastases present at the time of diagnosis [15,17]. This has two therapeutic consequences: (1) Treatment is local control (Surgery +/-Radiotherapy) only, while (2) Cure is predetermined by the extent of prior dissemination. Cure rate is increased by attacking putative preexistent micrometastases which cannot be detected, with adjuvant systemic therapy (hormonal therapy and/or cytotoxic chemotherapy). This is corroborated by the observation that 50% of women with operable breast cancer who receive locoregional treatment alone will die from metastatic disease [3,18].
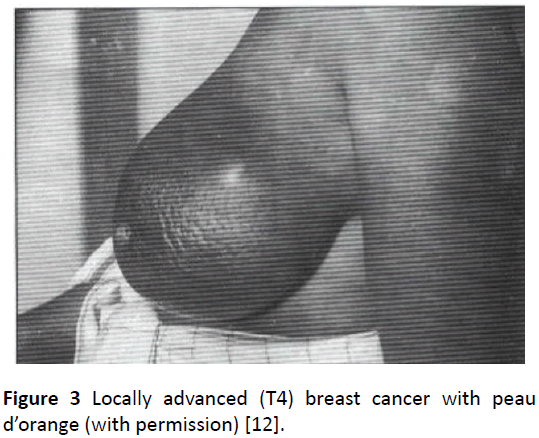
The International (Union International Contre le Cancer) TNM classification of breast cancer: T=Tumour. Tis: carcinoma in situ (pre-invasive), Paget’s disease (no palpable tumour); T0: no clinical evidence of primary tumour; T1: 0-2 cm, no skin fixation; T2: 2-5 cm, skin distortion, no pectoral fixation; T3: 5-10 cm, skin ulcerated over the lump, pectoral fixation and T4: tumour larger than 10 cm, breast skin involved beyond the lump, oedema, lymphocytic infiltration, ulceration of skin or satellite nodules, chest wall fixation. N=Nodes. N0: no palpable ipsilateral axillary lymph nodes nodes; N1: palpable mobile nodes (a) inflammatory only, (b) containing tumour, N2: palpable immobile ipsilateral axillary nodes; N3 (M1): ipsilateral supraclavicular or infraclavicular nodes, oedema of the arm. M=Metastases. M0: no metastases; M1: evidence of metastasis including skin beyond the breast, contralateral breast cancer, nodes, liver, bone, etc. For example, T2 N0 M0 is a malignant mass less than 5 cm in diameter causing puckering of the skin on elevation of the arm but no other abnormalities found [14]. Early breast cancers (T0-3 N0-1 M0) are operable cancers. They are confined to the breast and regional axillary lymph nodes and therefore considered potentially curable [19,20]. Late breast cancers (T4 N2/3 MI) are not curable as they are advanced tumours (Table 3 and Figure 3). Because of distant spread cure is remote. The aim is to prolong survival as far as possible and prevent undue suffering. The median survival is 14-20 months and palliation is all that can be achieved [21,22]. The significance of clinical staging of breast cancer is illustrated in Table 3 [23].
Figure 3: Locally advanced (T4) breast cancer with peau d’orange (with permission) [12].
| UICC stage | TNM | Category | 5-year survival (%) |
|---|---|---|---|
| I | T1, NO, M0 | Early cancer (operable, curable) |
84 |
| II | T1, N1, M0 T2, N01, M0 |
Early cancer (operable, curable) |
71 |
| III | Any T, N2-3, M0 T3, any N, M0 |
Locally advanced breast cancer (Neoadjuvant treatment to make operable) |
48 |
| IV | Any T, any N, M1 | Metastatic (Not curable, Inoperable, Palliative care) |
18 |
Table 3: Stage and prognosis according to TNM Classification.
Imaging
The effectiveness of any imaging technique depends on: the optimal demonstration on the breast, recognition of any abnormality and an accurate interpretation of the change. Optimal imaging requires familiarity with the techniques available and their limitations. The breast surgeon needs to know whether the image is normal, abnormal or equivocal. Most problems in imaging concern equivocal appearances and what to do about them. A sound knowledge of established and recent advances will help in selection of appropriate further investigations.
Mammography
X-ray mammography remains the most sensitive method for the detection of small breast cancers. Findings of X-ray mammography should always be considered together with clinical signs, and where appropriate results of needle biopsy (fine needle aspiration cytology, FNAC, or core biopsy, CB) when deciding management. In addition to its role in the ‘triple assessment’ of breast cancer, the aim of imaging (mammography/ ultrasonography) is not to confirm the diagnosis but to simply demonstrates whether there is single focus disease or not, coexisting DCIS, and, if there is synchronous contralateral breast disease [2,13,24,25]. This would influence the choice of surgery as to whether breast conserving or mastectomy is appropriate [2,26]. The variation in the appearance of the normal breast is due to the normal variation in the proportion of glandular tissue and fat. The dense appearance of the breast in younger women below 35 is due to the greater proportion of glandular tissue and may make cancers more difficult to demonstrate in this group. The less dense breast tissue in older women increases the sensitivity and specificity of mammography especially in demonstrating the margins of the tumour. In the investigation of women with symptomatic breast disease and in the assessment of screening detected abnormalities, the imaging investigation will often involve ultrasound (US). Breast specialists need to be familiar with the principal abnormal mammographic signs (mass, microcalcification, spiculation, architectural distortion or stellate lesions, asymmetry), together with their differential diagnosis and further methods of investigation [11,24,25,27].
Ultrasound
Ultrasonography (US) is a recognised component of the ‘triple assessment’ of breast lump, most useful in women less than 35 years old as their breasts are dense and not suitable for mammography and in the further evaluation of a mammogram [11]. A typical carcinoma is seen as an ill-defined mass with irregular, speculated margins and heterogenous internal echoes, distal irregular (acoustic) shadowing-in contrast to the typical appearance of a benign cyst or fibroadenoma [11,28]. Additional features may include a ‘halo’ of increased echogenicity and distortion of the surrounding normal breast parenchyma.
Diagnostic role: Ultrasound is very useful for confirming the diagnosis of malignancy, but cannot be used to definitively differentiate benign from malignant solid lesions in the breast. The use of US in the breast clinic reduces the number of unnecessary biopsies for clinically or mammographically suspicious lumps by 6%. It is also useful when attempting to aspirate small, deeply sited cysts. These may be missed by a fine needle without the US to guide the breast specialist. As a result, fewer unnecessary benign biopsies are performed following the introduction of US scanner into the clinic. Overall, when used for the diagnosis of malignancy, breast US has a sensitivity of 72%; a specificity of 97%; and a positive predictive value of 79%. US may be the only modality to suggest malignancy in less than 3% of patients with palpable lumps, where mammography and FNAC are normal [2,28]. Not all breast cancers are detectable by mammography and ultrasonography. Persistence of a lump or other clinically suspicious area necessitates further investigation. The common pitfall is the lobular type of breast cancer which may present as diffuse lumpiness rather than a discrete lesion [25].
Preoperative staging of the axilla: As 30%-40% of patients with early breast cancer have nodal involvement and clinical evaluation of the axilla is unreliable, pre-treatment ultrasound evaluation of the axilla should be performed for all patients being investigated for early breast cancer. If morphologically abnormal lymph nodes are identified, U/S-guided needle sampling should be offered. However, it is not 100% reliable [2]. Histological examination of resected tissue remains the only reliable method for determining axillary nodal status [29].
Therapeutic role: US can be used for the accurate measurement of breast cancers and their response to systemic therapy such as tamoxifen in elderly or infirm patients.
Magnetic Resonance Imaging
Diagnostic role
MRI of the breasts is offered to patients with invasive breast cancer (a) if there is a discrepancy regarding the extent of diseases from clinical examination, mammography and ultrasound assessment for planning treatment. The assessment of locoregional extent of breast cancers is more accurate with MR than mammography [30,31]; (b) if breast density precludes accurate mammographic assessment, (c) to assess the tumour size if breast conserving surgery is being considered for invasive lobular cancer, (d) in the assessment of multifocal/bilateral disease and in patients with prosthesis (for prosthetic rupture or detection of underlying tumour [28]. High sensitivities for detection of recurrent disease in conserved and irradiated breasts have been reported. There is however, as yet no evidence to support any role for breast MR in the breast screening programme [31].
Limitations
Most series have reported sensitivities of over 90% for detection of breast cancers using contrast-enhanced MR but the major problem is inadequate specificity [30]. Attempts to improve this have largely concentrated on analyses of rates and patterns in contrast enhancement. However, the overlap of enhancement behaviour of benign and malignant lesions limits this approach. The development of a breast cancerspecific contrast agent may improve technique specificity.
Therapeutic applications
These include the development of MR-guided breast biopsy and therapies to breast tumours (radio frequency and laser application, cryotherapy and focused ultrasound) [30,31].
Pathology
The pathological findings would confirm the diagnosis. The two main methods which have evolved for the establishment of a preoperative diagnosis in breast disease are fine needle aspiration which yields a cytological specimen (FNAC), and wide bore needle core biopsy which yields a histological preparation. Both methods have been shown to produce excellent results in symptomatic practice and have subsequently been applied successfully to the evaluation of mammographic screen- detected lesions. The safest way to use FNAC and core biopsy in preoperative diagnosis is by employing the ‘triple approach’. This concept combines the results of clinical examination, imaging (mammography and ultrasound) and FNAC or core biopsy. When all three agree, the level of diagnostic accuracy exceeds 99% [8-10]. There are a number of advantages in obtaining a preoperative diagnosis in women with abnormalities of the breast. For the patient, the reduction in the number of unnecessary open biopsies for benign disease, and the opportunity for early counselling for appropriate therapy in cases were a preoperative diagnosis of malignancy is established in the same breast clinic would minimise anxiety; For the surgeon, the reduction in the number of unnecessary operations for benign disease which results in better planning of operating lists and bed occupancy; For the pathologist the reduction in the number of unnecessary and time-consuming frozen sections [2,3,12].
Fine needle aspiration cytology (FNAC)
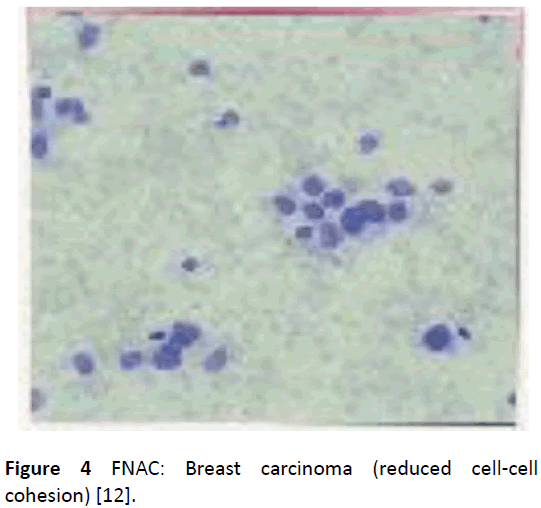
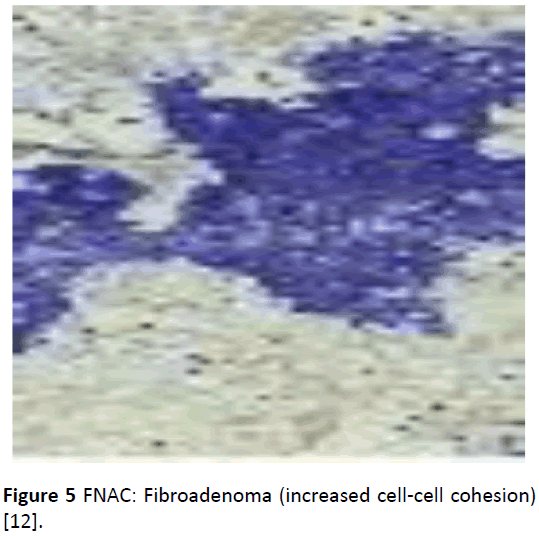
FNAC of lump confirms the diagnosis immediately thus allowing an informed discussion with the patient about treatment options. It also reassures the patient. The advantage of doing an FNAC prior to a mammogram is that the patient obtains a definitive diagnosis without waiting. The disadvantage is that it can be painful doing a mammogram immediately after an FNAC. It may also obscure the reading of the mammogram, [3,32]. However, in breast centres where a mammogram is readily available a mammogram before FNAC or core biopsy is routine although timing is necessary. The cytology grading is as follows: C1: insufficient cells, C2: normal cells, C3: benign cells, C4: suspicious, C5: malignant cells. The sensitivity will always be less than 100%, i.e., there will be some false negatives, but the specificity should be 100% for a good cytopathologist i.e. there should be no false positives. If it is breast cancer, it is breast cancer [2,5,32]. In carcinoma cell populations, cell-to-cell cohesion is reduced and it is often possible to get a high yield from an aspirate sample (Figure 4), whereas the arrangement of benign cells as in fibroadenoma are in large tightly cohesive groups (Figure 5). A C5 report in conjunction with clinical and radiological evidence of a carcinoma is sufficient evidence to proceed to definitive surgery with curative intent [2,32]. Fine needle aspiration (FNA) is also useful in revealing the consistency of the lump, the gritty feel of carcinoma or the fluctuant nature of a cyst. A cyst can be treated by FNA, if blood-stained FNAC is done. If there is a residual mass after aspiration an FNAC of the mass is done. If cysts refill re-aspiration is done and if cysts persistently refill or cytology is suspicious an excision biopsy is done. It is advisable to do fine needle aspiration cytology of a clinically diagnosed fibroadenoma if greater than 2.5 cm, or in a patient with significant family history of breast carcinoma, or in a patient over the age of 25 years. Smaller lesions do not warrant excision but the patient should be reassured and followed-up [2]. Other uses include smearing nipple discharge and performing FNAC of an underlying lump and FNAC and skin biopsy (for Paget cells) of nipple eczema. Ductal ectasia and fat necrosis following trauma may cause nipple retraction or skin tethering and so FNAC or excision biopsy will be useful to exclude carcinoma. Generally, if cytology is suspicious or does not fit with clinical examination, one can go further and do either a core biopsy or an excision biopsy.
Figure 4: FNAC: Breast carcinoma (reduced cell-cell cohesion) [12].
Figure 5: FNAC: Fibroadenoma (increased cell-cell cohesion) [12].
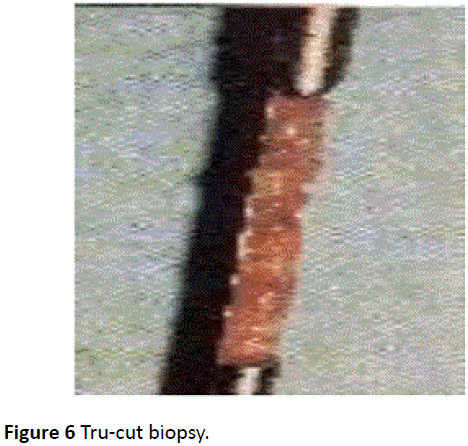
Core biopsy
It is a histopathological diagnosis as a core of tissue is examined. It is usually a tru-cut or needle biopsy but the sensitivity is however still less than 100% (Figure 6) [33-35]. It is still higher than FNA as it yields adequate samples especially from solid lumps. Definite diagnosis of specific benign lesions e.g. fibroadenoma may be possible. It is also much better than FNAC in diagnosing pre-invasive in-situ carcinoma in the breast (carcinoma cells which have not yet broken through the basement membrane) especially screened-detected [36]. Microcalcifications are more readiliy identified than with FNAC and the cores should be X-rayed if microcalcifications were present on the original radiograph. Generally, core biopsy should not be used instead of FNAC but it is a valuable adjunct [2,37].
Excision biopsy
It is a histopathological diagnosis and, most assuring as the whole lump is removed (Figure 7). The sensitivity is 100% [38-40]. The incision should be made in the direction of the skin crease (Langer’s lines) of the breast to avoid the formation of a hypertrophic or keloid scar (Figure 8) [41].
Staging of Breast Cancer
Following a definitive diagnosis of breast cancer from the triple assessment, staging of the disease is necessary.
Metastatic work up
In the 1980s extensive staging investigations were carried out including radioisotope bone scanning and ultrasound liver examination. The low yield of positive results from such tests and the realisation that up to a third of ‘early’ cancers will have tumour cells detectable in the bone marrow [3,42] has led surgeons to abandon superstaging. Instead, a few investigations are required to detect distant metastases (i.e. limited staging).
These are (a) biochemical; (1) liver function test (alkaline phosphatase, alanine/aspartate transaminase) to detect the effect of liver metastases, (2) serum calcium to detect the effect of bony metastases; and (b) radiological; (1) chest X-ray to detect lung metastases or pleural effusion, (2) ultrasound scan of the liver detects liver metastases [2,3]. Only if these investigations are found to be abnormal is skeletal X-rays or occasional MRI necessary to detect bony metastases [2,3]. Patients with large (>4 cm) tumours or who are symptomatic (e.g. back pain) should have radioactive bone scans or liver ultrasound scans performed if a positive result would lead to a change in management.
Sentinel node biopsy
Sentinel node biopsy (a technique used to identify the first nodes (at least 2, that tumour drains to) is a more sensitive method of staging the axilla [43]. It accurately stages the axilla without the morbidity of axillary clearance. It is therefore useful for clinically node negative axilla and in early breast cancer (T1,T2). The lymph nodes can be located following the injection of either radioisotope, blue dye or both in peritumoral, subdermal or subareolar sites. It allows more detailed examination of nodes removed but the significance of micrometastatic deposits identified is still uncertain. It has a 15% false negativity rate [44]. Surgical evaluation of the axilla is still important and should be considered for all patients with invasive disease [29,45].
Prognostic Factors
Various pathological measurements influence prognosis, either individually or in combination. Prognostic factors have three main uses. (1) to select appropriate adjuvant therapy according to prognosis, (2) to allow comparison of treatment between similar group of patients at risk of recurrence or death, (3) to improve the understanding of the disease. Prognostic factors can be chronological or biological [46].
Chronological prognostic factors
They are an indication of how long the disease has been present and relate to the stage of the disease at presentation (TNM). (1) Age: younger women have poorer prognosis at equivalent stage. Hence, here the biology of the tumour is more important; (2) Tumour size: the diameter of tumour correlates directly with survival; (3) Lymph node status: is the single best prognostic factor. There is a direct correlation between number and level of nodes involved and survival [47].
Biological prognostic factors
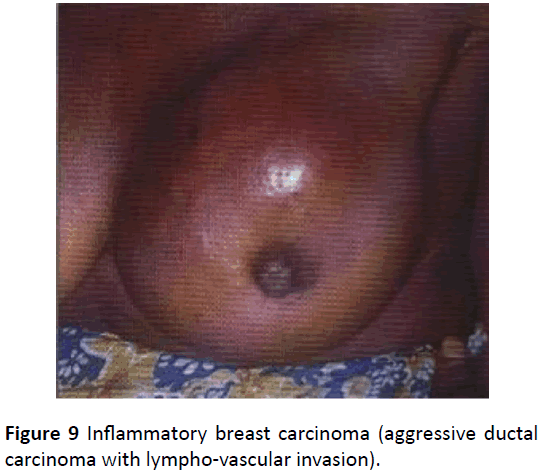
It relates to the intrinsic behaviour of the tumour and all have prognostic significance. (1) Histological type: some histological types are associated with improved prognosis: tubular, cribiform, mucinous, papillary and microinvasive. (2) Histological grade: Three characteristics allow scoring of grades into grade 1 (low grade) well-differentiated; Grade 2 (intermediate grade) moderately differentiated; Grade 3 (high grade) poorly differentiated. The histological grade depends on tubule formation, nuclear pleomorphism and mitotic frequency and the increasing grade indicates the likeliness to grow more quickly and to spread. (3) Lymphatic/vascular invasion: 25% operable breast cancers have lymphovascular invasion [48,49]. There is a double risk of local relapse and a higher risk of short term systemic relapse thus the need for neo-adjuvant chemotherapy as in inflammatory breast carcinoma (Figure 9) [46].
Biochemical measurements
Hormones, Growth factor, HER 2 receptors, Oncogenes: A sample of the breast tissue will usually be tested to see if it has these receptors and these will affect the type of treatment needed [13,18]. Oestrogen receptors and to a lesser extent progesterone receptors determine the response to endocrine therapy given either as treatment for locally advanced O metastatic breast cancer or given as an adjuvant after locoregional therapy [4,50]. Oestrogen receptor (ER) positivity predicts a 50%-70% chance of responding to hormonal therapy and this increases to over 70% in patients whose tumours have both oestrogen and progesterone receptors [2,21,46]. Usually cancers that are ER positive will also be progesterone (PR) positive. Epidermal growth factor (EGF) receptors are negatively correlated with oestrogen receptors and of poorer prognosis. Some early and advanced cancers have receptors for human epidermal growth factor type 2 (HER2) and tumours with high levels of these receptors (HER2 positive) may respond to targeted molecular (biologic) therapy with the monoclonal antibody e.g. trastuzumab (terceptin) [17,51]. Some breast cancers don’t have receptors for oestrogen, progesterone or HER2. This type of breast cancer is known as triple negative breast cancer and after surgery, chemotherapy is the main adjuvant treatment [2,52].
Oncogenes: Tumours that express C-erB2 oncogene are likely to be resistant to cyclophosphamide, methotrexate and 5-fluorouracil (CMF) chemotherapy and hormonal treatment but will respond to anthracycline and taxols [52,53]. The BRCA1-associated breast tumour has a worse prognosis than BRCA2-associated breast cancer [54-56]. They are of high grade, associated with negative ER-PR receptors, increased S phase fraction and aneuploidy and necessitates a contralateral prophylactic mastectomy [57,58].
Proteases: The presence of urokinase and cathepsin D confers poorer prognosis (17).
The Nottingham Prognostic Index
It has been shown to have good discriminant function as it is based on a combination of (1) tumour size, (2) histological grade and (3) axillary nodal status [46]. In the excellent prognostic group (EPG) which entails 10% of symptomatic patients and 30% of screened patients, survival is closed to age-matched controls. There is a 70% 5 yr survival and 30% 15 years survival in the moderate prognostic group (MPG), and, very poor outlook (die within 1 yr) in the poor prognostic group (PPG). It should be noted that a small tumour may be of high grade (poorly differentiated and aggressive) with a propensity of having micrometastases at time of diagnosis and vice versa [16]. The predictions of survival in an individual are relevant to the choice of adjuvant systemic therapy. In the excellent prognostic group (EPG) chemotherapy is not required; in the moderate prognostic group (MPG) hormonal or chemotherapy is rendered and in the poor-prognostic group (PPG) either no therapy or aggressive chemotherapy is rendered if patient is fit [46].
Factors: NPI=(0.2 x size (cm)+lymph node stage+tumour grade (Table 4).
| Involved nodes | Tumour grade | Score per factor |
|---|---|---|
| 0 | I | 1 |
| 1-3 | II | 2 |
| >3 | III | 3 |
| Prognosis | NPI score | Survival (15 yrs) (%) |
| Good | <3.4 | 80 |
| Moderate | 3.4-5.4 | 40 |
| Poor | >5.4 | 15 |
Table 4: Nottingham Prognostic Index (NPI) [46].
Screening
Screening is the search for unsuspected disease in a population of apparently healthy people. The hypothesis is that the detection and treatment of cancers at an asymptomatic stage enables the cure of lesions which would be incurable if left until patients present with symptoms [59]. However, the natural history of breast cancer is renowned for its variability in growth rates. Some lesions progress very slowly and would probably be curable even if left until they are symptomatic. Other lesions may progress rapidly to incurability even before they are detectable by screening. It is for the group of breast cancers in between these two extremes that screening would be valuable. Local treatment of breast cancer at a symptomatic stage failed to provide long-term control. This was due to early dissemination with the production of micrometastatic disease. It was known that there was a recognizable pre-clinical phase during which such dissemination was less likely to have occurred, and that it could be detected by mammography [60,61]. The Health Insurance Plan study in New York first demonstrated a significant reduction in the mortality from breast cancer detected by mammographic screening. These findings were corroborated by trials in the Netherlands and Sweden [62]. Following the Breast Cancer Screening report by a working party chaired by Sir Patrick Forrest in 1987, the UK National Breast Screening Programme was established offering single view mammography every 3 years to women aged 50-64 [7]. Eight controlled randomized trials including over 500,000 women have confirmed the validity of this approach. Further two randomized control trials and two case-controlled studies had indicated that in women over 50 years of age, mammographic screening led to prolongation of life [60,61]. The effectiveness of any screening programme is enhanced when a higher risk population is screened. Trials of mammographic screening have failed to demonstrate a reduction in the mortality rate in women under the age of 50 years [63]. However, after ten years of follow-up a nonsignificant trend towards reduced mortality ranging from 13% to 23% has been reported in several studies [64]. A recent meta-analysis demonstrated no reduction in breast cancer mortality in women aged 40-90 years. A major concern regarding the studies is that the individual studies included a relatively small number of women aged 40-49 years. Thus, the ongoing debate on the value of mammographic screening in women under 50.
The acceptability of mammography is indicated by high uptake rates. The sensitivity of the test is evidenced by the fact that well over 90% of cancers are detectable by mammography. The specificity of mammography is shown by the less than 1: 1 ratio of benign to malignant biopsies of detected lesions [2]. A possible disadvantage of inviting women for breast cancer screening is that a degree of anxiety may be generated in the population. This reduced by keeping recall rates for repeat mammography to less than 10% by quality control. The risk of single view mammography itself inducing a fatal breast cancer is estimated to be as small as 1 in 100,000 [64,65].
Screening advances the date of diagnosis thereby extending the time between diagnosis and death even if this was unaltered (lead time bias). Slow growing cancers are more likely to be detected in their longer asymptomatic growth phase (length bias). Women who attend for screening are also likely to be health conscious and to seek treatment early when symptomatic, while those who do not attend may ignore early symptoms and be at higher risk of dying from breast cancer (selection bias) [6,7,50]. Nevertheless, meta-analysis of published studies of breast screening suggests that mortality can be reduced by a quarter in the age group screened by the national programme. More than a fifth of the tumours detected annually were less than 1 cm in diameter and impalpable [6,7]. Breast conserving operations and adjuvant systemic therapy have enhanced the acceptability and effectiveness of the treatment offered to screening detected cancers [50].
Interval cancers
Interval cancers defined as breast cancers diagnosed in the interval between scheduled screening episodes. They are inevitable in any screening programme but their numbers should be kept to a minimum [65]. A high proportion of interval cancers in a screening programme will reduce the likelihood of achieving a mortality reduction in the population being offered screening. Review and sub-classification of interval cancers is an essential part of routine radiological audit. The need to conduct research into the best form of screening was recognised. Three randomized trials were set up to determine the optimum method of screening including computer-assisted digital analysis of mammographic abnormalities, the optimum frequency and the value of screening in younger women. It was however, recognized that a screening programme was not a diagnostic test and resources were provided for the performance of these further tests within the service [64,65].
The surgeon’s and pathologist’s role
Most screen-detected patients requiring surgery will have an impalpable abnormality. A very few will report a palpable lump at screening, who will be brought back as ‘symptomatic recalls’ despite having a normal mammogram.
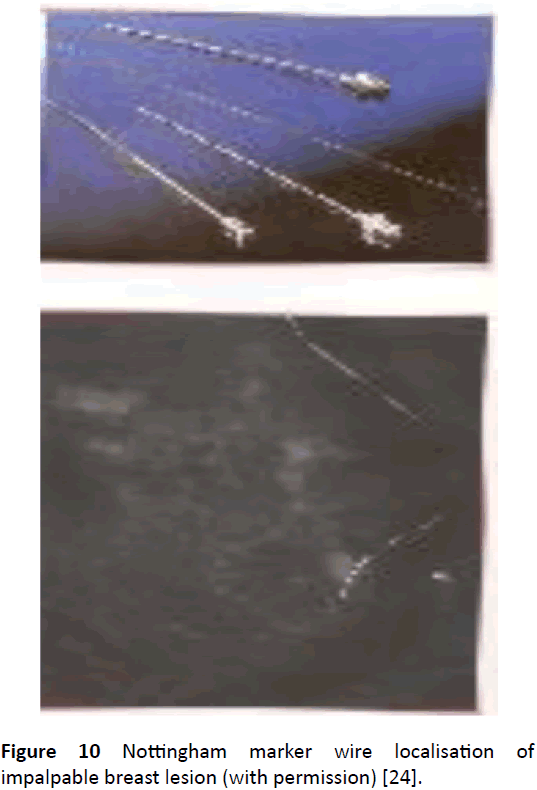
The nature of the surgical procedure carried out will depend on the amount of pre-operative information gathered during the assessment process. The material available for cytological and histological examination will be a major factor in determining whether the surgery is diagnostic or therapeutic. Fine needle aspiration cytology (FNAC) can confirm the presence of malignant cells (C5), but cannot differentiate in situ from invasive disease with accuracy [66]. Cores of tissue obtained with a core biopsy (CB) needle are required to make this decision with confidence [67]. Impalpable lesions will require the stereotaxic placement of a guide wire, or the use of US to facilitate their localisation prior to removal (Figure 10) [11,67,68]. The availability of a mammographic screening service has also had a marked effect on the management of women attending hospital with symptomatic breast disease [2,3].
Figure 10: Nottingham marker wire localisation of impalpable breast lesion (with permission) [24].
Conclusion
The preoperative diagnosis of breast cancer would be established in over 90% of palpable early cancers if the screening programme and the symptomatic guidelines entailing ‘triple assessment’ are implemented. In addition to the improved diagnostic yield of using the triple diagnostic modalites of clinical examination, radiological and cytopathological assessment the rapid pre-operative diagnosis would allow the patient to come to terms with the enormous psychological effects of a cancer diagnosis and with adequate counselling about treatment options. The outcome of treatment is predetermined by the intrinsic nature of the tumour and the presence of putative micrometastases. Therefore although the TNM staging system is still widely used, of more importance is the full pathological description which allows patients to be compared in a meaningful manner. The combination of tumour size, grade and nodal status (Nottingham prognostic index) has been shown to have to have good discriminant function. On the basis of clinical staging (TNM), however, a decision can be made on whether to attempt cure or palliation. In early breast cancer (T0-2(3), N0-1, M0), with apparently no distant metastases cure can be attempted bearing in mind that the prognosis is related to the intrinsic nature of the tumour. In late breast cancer (T4, N2, M1) no cure is possible because of disseminated disease. Although screening demonstrates a reduction in mortality rate by a quarter in women over the age of 50 years, the shortcomings of lead-time, length and selection biases, interval cancers and women under 50 years remain.
References
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, et al. (2009) Cancer statistics? CA Cancer J Clin 59: 225-249.
- Catterall A (1995) Guidelines for surgeons in the management of symptomatic breast disease in the United Kingdom. Eur J Surg Oncol 21: 1-13.
- Bundred NJ, Downey SE (1996) The management of early breast cancer. Curre pract Surg 8: 1-6.
- Baum M, Houghton J (1999) Contribution of randomised controlled trials to understanding and management of early breast cancer. Br Med J 319: 568-571.
- Smallwood J, Herbert A, Guyer P, Taylor I (1985) Accuracy of aspiration cytology in the diagnosis of breast disease. Br J Surg 72: 841-843.
- Tabár L, Fagerberg CJ, Gad A, Baldetorp L, Holmberg LH, et al. (1985) Reduction in mortality from breast cancer after mass screening with mammography. Randomized trial from the breast cancer screening working group of the Swedish National board of Health and Welfare. Lancet 1: 829-832.
- Working party on breast cancer screening (1986) Report to the Health Minister of England and Wales, Scotland and Northern Ireland. London: HMSO.
- Van Bogaert LJ, Mazy G (1977) Reliability of the cyto-radio-clinical triplet in breast pathology diagnosis. Acta Cytol 21: 60-62.
- Thomas JM, Fitzharris BM, Redding WH, Williams JE, Trott PA, et al. (1978) Clinical examination, xeromammography, and fine-needle aspiration cytology in diagnosis of breast tumours. Br Med J 2: 1139-1141.
- Hermansen C, Poulsen HS, Jensen J, Langfeldt B, Steenskov V, et al. Diagnostic reliability of combined physical examination, mammography, and fineÃÆâÃâââ¬ÃâÃÂneedle puncture (“tripleÃÆâÃâââ¬ÃâÃÂtest”) in breast tumors: A prospective study. Cancer 60: 1866-1871.
- Blamey R, Evans A, Ellis I, Wilson R (1999) Atlas of Breast cancer. Merit publishing international Basingstoke, UK.
- Weledji EP, Enoworock G (2012) Breast cancer: clinical diagnosis and assessment- a review. J Med Medi Science 3: 601-609.
- Dixon MJ, Mansel RE (1992) The breast. In: Burnand KG, Young AE (eds) The New Aird’s companion in surgical studies. Edinburgh: Churchill Livingstone 811-844.
- Sobin LH (2009) International Union Against Cancer (UICC) TNM classification of malignant tumours. Oesophagus including Oesophagogastric Junction 66-72.
- Fischer B (1980) Laboratory and clinical research in breast cancer. A personal adventure. The David A Karnofsky memorial lecture. Cancer Res 40: 3863-3874.
- Barr LC, Baum M (1992) Time to abandon TNM staging of breast cancer? Lancet 339: 915-917.
- Fischer B (1999) From Halstead to prevention and beyond. Advances in the management of breast cancer during the twentieth century. Eur J Cancer 5: 1963-1973.
- Early breast cancer Trialists’ collaborative group (1992) Systemic treatment of early breast cancer by hormonal, cytotoxic or immune therapy.133 randomised trials involving 31000 recurrences and 24000 deaths among 75000 women. Lancet 339: 1-16.
- Veronesi U (1998) Treatment of primary breast cancer. Ballieres Clin Oncol 2: 175-193.
- Whitman GJ, Strom EA (2009) Workup and staging of locally advanced breast cancer. Semin Radiat Oncol 19: 211-221.
- Scholl SM, Fourquet A, Aselain B, Pierga JY, Vilcoq JR, et al. (1994) Neoadjuvant versus adjuvant chemotherapy in premenopausal patients with tumours considered too large for breast conserving surgery: preliminary results of a randomized trial. Eur J Cancer 30: 645-652.
- Ogundiran TO, Ayandipo OO, Ademola AF, Adebamowo CA (2013) Mastectomy for management of breast cancer in Ibadan, Nigeria. BMC Surg 13: 59.
- Weledji EP, Elong FA (2017) Primary surgical treatment of locally advanced breast cancer with heavy nodal involvement: a case report. International J Surg Oncol 2: e08.
- Tabar and Dean (1985) Teaching Atlas of Mammography. Georg Thieme Verlag: New York, Thieme Med Pub.
- Sainsbury JRC (1996) Breast cancer. Postgraduate Med J 72: 663-666.
- Veronesi U, Banfi A, Slavadori B, Luini A, Saccozzi R, et al. (1990) Breast conservation is the treatment of choice in small breast cancer: longer term results of a randomized trial. Eur J Cancer 26: 668-670.
- Muirr BB, Lamb J, Anderson TJ, Kirkpatrick AF (1983) Microcalcification and its relationship to cancer of the breast: Experience in a screening clinic. Clin Radiol 34: 193-200.
- Jokich PM, Monticciolo DL, Adler YT (1992) Breast Ultrasonography. Radiol Clini North America 30: 993-1009.
- Taylor I (1995) How should the axilla be treated in breast cancer? Eur J. Surg. Oncol 21: 2-7.
- Davidson T, Mumatz H, Hall-Craggs MA, Kissin MW, Thurell W, et al. (1997) The impact of magnetic resonance imaging in determining surgical management in breast cancer. The Breast 6: 177-182.
- Mumatz H, Hall-Craggs M, Davidson T, Walmsley K, Thurell W, et al. (1997) Staging of symptomatic primary breast cancer with MR imaging. Am J Radiol 169: 417-424.
- Cytology Sub-group, of the National Coordinating Committee for Breast Screening Pathology (1992) Guidelines for cytology Procedures and Reporting in Breast Cancer screening. Sheffield: NHS Breast Screening Programme.
- Elston CW, Cotton RE, Davies CJ, Blamey RW (1978) A comparison of the use of the ‘Tru-cut’ needle and fine needle aspiration cytology in the pre-operative diagnosis of carcinoma of the breast. Histopathol 4: 239-254.
- Fentiman IS, Millis RR, Hayward JL (1980) The value of needle biopsy in out-patient diagnosis of breast cancer Arch Surg; 115: 652-653.
- Minkowitz S, Moskowitz R, Khafif RA, Alderete MN (1986) TRU-CUT needle biopsy in the diagnosis of carcinoma of the breast. An analysis of its specificity and sensitivity. Cancer 57: 320-323.
- Cusick JD, Dotan J, Jaecks RD, Boyle WT Jr. (1990) The role of Tru-Cut needle biopsy in the diagnosis of carcinoma of the breast. Surg Gynecol Obstet 17: 407-410.
- Vega A, Garijo F, Ortega E (1994) Core needle aspiration biopsy of palpable breast masses. Acta Oncol 34: 31-34.
- Parker SH, Burbank F, Jackman RJ, Aucreman CJ, Cardenosa G, et al. (1994) Percutaneous large-core breast biopsy: a multi-institutional study. Radiology 193: 359-364.
- Gisvold JJ, Goellner JR, Grant CS, Donohue JH, Sykes MW, et al. (1994) Breast biopsyg: a comparative study of stereotaxically guided core and excisional techniques. Am J Roentgenol 162: 815-820.
- Bentley PG (1989) A simple specimen mount for the orientation of breast biopsies. Br J Surg 76: 312.
- Weledji EP, Ngwane S (2002) The management of keloids and hypertrophic scars. Darlin Count Durh Med J 16: 39-45.
- Mansi JL, Berger I, Easton D, McDonnell T, Redding WH, et al. (1987) Micrometastases in bone marrow in patients with primary breast cancer. Evaluation as an early predictor of bone metastases. Br Med J 295: 1093-1096.
- McIntosh SA, Purushotham AD (1998) Lymphatic mapping and sentinel node biopsy in breast cancer. Br J Surg 85: 1347-1356.
- Noguchi M (2002) Sentinel lymph node biopsy and breast cancer. Br J Surg 89: 21-34.
- Fentiman IS, Mansel RE (1991) The axilla: not a no-go zone. Lancet 337: 221-223.
- Galea MH, Blamey RW, Elston CE, Ellis IO (1992) The Nottingham prognostic index in primary breast cancer. Breast Can Res Treat 22: 207-219.
- Veronesi U, Galimberti V, Zurrida S, Merson M, Greco M, et al. (1993) Prognostic significance of number and level of axillary node metastases in breast cancer. Breast 2: 224-228.
- Elston CW, Ellis IO (1991) Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 19: 403-410.
- Dalton IW, Page DL, Dupont WD (1994) Histologic grading of breast carcinoma: a reproducibility study. Cancer 73: 2765-2770.
- Shapiro CL, Henderson IC (1994) Adjuvant therapy of breast cancer. Haematology/ Oncology Clinics of North America 8: 213-231.
- Coleman R (1996) The management of advanced breast cancer. Curr Pract Surg 8: 7-12.
- Hortobagyi GN (2005) Trastuzumab in the treatment of breast cancer. N Eng J Med 353: 1734-1736.
- Dixon MJ, Leonard RCF (1997) Hormones and chemotherapy. In: Breast and Endocrine surgery (editor: JR Farndon). A companion to specialist surgical practice. WB Saunders comp Ltd London.
- Fitzgerald MG, MacDonald DJ, Krainer M, Hoover I, O'neil E, et al. (1996) Germ-line BRCA1 mutations in Jewish and non-Jewish women with early- onset breast cancer. New Engl J Med 1996 334: 143-149.
- Langston AA, Malone KE, Thompson JD, Daling JR, Ostrander EA (1996) BRCA1 mutations in a population- based sample of young women with breast cancer. New Engl J Med 334: 137-142.
- Tonin P, Weber B, Offit K, Couch F, Rebbeck TR, et al. (1996) Frequency of recurrent BRCA1 and BRCA2 mutations in Ashkenazi Jewish breast cancer families. Nat Med 2: 1179-1183.
- Easton D, Peto J (1990) The contribution of inherited predisposition to cancer incidence. Cancer Surv 9: 395-416.
- Claus EB, Risch NJ, Thompson WD (1990) Age at onset as an indicator of familial risk of breast cancer. Am J Epidemiol 131: 961-972.
- Bates T (1996) Screening for malignant disease. In: Clinical Surgery in general RCS Course manual. Kirk, Mansfield and Cochrane (eds); Churchill livingstonr; chp 22.
- Verbeek AL, Holland R, Sturmans F, Hendriks JH, Avunac M, et al. (1984) Reduction of breast cancer mortality through mass screening with modern mammography. First results of the Nijmegen project, 1975-1981. Lancet 1: 1222-1224.
- Waard FD, Collette HJ, Rombach JJ, Day NE (1984) Evaluation of screening for breast cancer in a non-randomised study (the DOM project) by means of a case-control study. Lancet 1: 1224-1226.
- Tabor L, Gad A, Holmberg LH, Ljungquist U, Fagerberg CJ, et al. (1985) Reduction in mortality from breast cancer after mass screening with mammography. Randomized trial from the breast cancer screening working group of the Swedish National board of Health and Welfare. Lancet 325: 829-832.
- Fletcher S (1997) Breast cancer screening in women under 50. Br Med J 314: 764-765.
- Kissin M (1983) Breast screening and screen-detected disease. In: Breast and endocrine surgery, chp 8, (ed) Farndon JR: A companion to specialist surgical practice. WB Saunders company, London.
- NHS Breast Screening Programme (1997) Quality assurance Guidelines for Radiologists. Scheffield: NHS BSP Publications 15.
- Lamb J, Anderson TJ, Dixon MJ, Levack PA (1987) Role of fine needle aspiration cytology in breast cancer screening. J Clin Pathol 40: 705-709.
- Dowlatshahi K, Taremko ML, Kluskens LF, Jokich PM (1991) Non-palpable breast lesion : findings of stereotaxic needle-core biopsy and fine-needle aspiration cytology. Radiology 181:745-750.
- Dahltrom JE, Jain S, Sutton T, Sutton S (1996) Diagnostic accuracy of stereotaxic core biopsy in a mammography breast cancer screening programme. Histopathology 28: 412-427.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences