Perspectives on Enterocutaneous Fistula: A Review Article
Elroy Patrick Weledji
DOI10.21767/2471-299X.1000047
Elroy Patrick Weledji*
University of Buea, Buea, Southwest Region, Cameroon
- *Corresponding Author:
- Elroy Patrick W
Faculty of Health Sciences, Bomaka Buea
Southwest Region, P.O. Box 63, Cameroon
Tel: +237699922144
E-mail: elroypat@yahoo.co.uk
Received date: May 22, 2017; Accepted date: June 08, 2017; Published date: June 15, 2017
Citation: Weledji EP (2017) Perspectives on Enterocutaneous Fistula: A Review Article. Med Clin Rev 3:5 doi: 10.21767/2471-299X.1000047
Copyright: © 2017 Weledji EP. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Patients with enterocutaneous fistula can almost always be cured nowadays, and if the right steps are adopted hospital death rarely occurs. A multidisciplinary approach is essential but investigating the fistula is unimportant in the early stages of management. The management is heavily influenced by the underlying aetiology and the anatomical classification of the fistula, which together determine the likelihood of resolution without surgical intervention. The aim of the study was to review the rationale and evidence basis behind the current management strategy of enterocutaneous fistula.
Keywords
Fistula; Enterocutaneous; Spontaneous; Postoperative (Iatrogenic); Management
Introduction
Enterocutaneous fistulas are abnormal communications between the gastrointestinal tract and the skin. They are associated with considerable morbidity and mortality compared with other surgical conditions. Sepsis was the leading cause of death and increased mortality has been shown to be associated with complications of high initial fistula output, increased Acute Physiology And Chronic Health Evaluation II (APACHE II) scores, low serum albumin and patient co-morbidity [1,2]. Sepsis and inflammatory bowel disease can add to the patient’s state of malnutrition with effect on fistula closure, and, weight loss is a basic indicator of surgical risk [3,4]. Much of the literature concerning enterocutaneous fistula are retrospective reviews usually originating from specialist canters with expertise in management. There are few large randomized controlled trials addressing optimal management and, therefore continues to present a considerable challenge. The goal is fistula closure with minimal morbidity and mortality. The current trend emphasizes on the initial focus of correcting the fluid and electrolyte disturbances, aggressive treatment of sepsis, control of fistula output and attention to skin care and psychological support [5]. Intake of fluid low in sodium is restricted and electrolyte solution containing high concentration of both sodium and glucose substituted. Parenteral nutrition in high output enterocutaneous fistulas has influenced fistula closure rates and mortality positively [6]. Most often for postoperative fistulas, further surgery is contemplated only if the fistula persists after conservative measures. This article reviewed the rationale and evidence basis behind the current management strategy of enterocutaneous fistula.
Fistula Form ation and Characterization
Enterocutaneous fistulae can arise spontaneously from an underlying area of diseased bowel such as Crohn’s disease, tuberculosis, typhoid, diverticular disease, prior radiotherapy etc., but commonly follow complications of abdominal surgery (iatrogenic) such as an anastomotic leak. It is estimated that 75-85% of enterocutaneous fistula form after operation as a result of bowel injury, inadvertent enterotomy and/or anastomotic leakage [7]. The incidence of fistula formation may therefore be influenced by the skill and experience of the surgeon as well as patient variables [2,8]. Fistula formation is more commonly associated with surgery in the presence of malignancy or inflammatory bowel disease, and with attempted division of dense adhesions [3,9].
Enterocutaneous (Iatrogenic) fistula
Intestinal anastomoses either fail for technical reasons (<72 hrs) or for biological reasons (days or weeks). Technical reasons include surgical error (failure to make the anastomosis watertight, construction under too much tension etc.) or wrong suture material. But much the commonest reason is that the tissues fail (biological failure). The reasons for biological failure are ischemia, tissue quality and sepsis. The ends that are joined together may be already ischemic as sometimes the surgeon overzealously ‘tidy’ the bowel ends and thus divides too much of the blood supply [10]. Much more commonly healthy, viable ends are joined together, but in the early postoperative period as the blood pressure falls or the blood becomes haemo-concentrated because of inadequate fluid replacement sludging occurs in the perianastomotic capillaries. The areas of bowel will infarct, over a number of days the dead patch will autolyse and finally perforate as a clinical anastomotic leak. With regard to tissue quality steroids, cancer, jaundice, Crohn’s disease, and radiotherapy would lead to poor healing and an increased chance of an anastomosis failing. Some perianastomotic sepsis is caused by anastomotic failure, but equally anastomotic failure can be caused by an abscess that ruptures back through the anastomosis [7]. Fistulas, whether postoperative (iatrogenic) or spontaneous, will drain along the line of least resistance, which is often previous scar tissue from incisions, drain sites, often through the main abdominal wound or directly through an area of un-operated skin [7,11,12]. Clinically there is evidence of sepsis but the patient will often feel rather better once the fistula has discharged, but shortly afterwards the other problems of an enterocutaneous fistula will emerge. Postoperative mortality from the sepsis of an anastomotic leak is higher (39.3%) than any natural condition [13]. Anastomotic leak was found to be an independent predictor of mortality corroborated by the fact that delayed diagnosis worsens the prognosis [13-15].
In the remaining 15-25% of instances, enterocutaneous fistulas form secondary to underlying pathology. Inflammatory bowel disease in particular Crohn’s disease is the commonest cause of spontaneous enterocutaneous fistulation in the developed world [2,7]. Other causes include radiation enteritis, diverticular disease, malignancy, intra-abdominal sepsis and trauma [16-18]. In developing countries, spontaneous fistulation complicate infectious conditions, such as abdominal tuberculosis, amoebiasis and typhoid [19].
Enterocutaneous spontaneous fistula
Enteric fistulas may affect up to 30% of patients with Crohn’s disease. The fistulae of Crohn’s disease may be internal (40%) or external (40%) or mixed (20%) and relate to extra intestinal sepsis due to the transmural inflammation and deep intestinal ulcers [20]. Penetrating ulceration of the diseased bowel may produce a contained bowel perforation and abscess although the septic component may be occult or minimal. Fistulae develop when the abscess necessitates into an adjacent viscus or through a cutaneous wound. Internal fistulas are usually spontaneous and external ones postoperative [21]. Intra-abdominal abscesses that are drained externally will usually result in an enterocutaneous fistula. Thus when abscesses complicate existing fistulas it is necessary to convert these fistula/abscess complexes into a well-draining controlled fistula [2,22]. Enterocutaneous fistulas in Crohn’s disease are a common cause of intestinal failure i.e. inability to maintain adequate nutritional, fluid and electrolyte homeostasis without supportive therapy, and patients who develop intestinal failure are best managed in a specialist unit with a multidisciplinary team [12].
Characterization
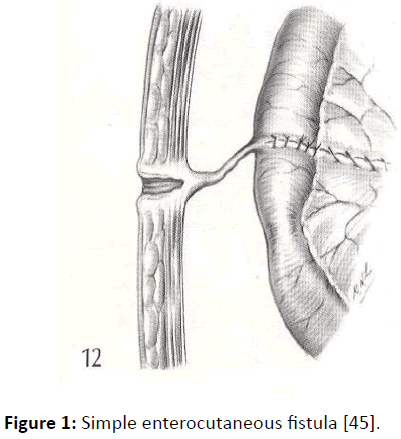
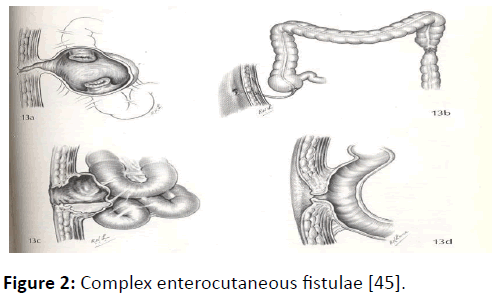
There is no universally accepted classification scheme for enterocutaneous fistulas. Characterization is usually made on the basis of anatomy (site of origin, simple or complex fistula, end or lateral fistula, presence or absence of distal obstruction) or fistula output (with high output usually defined as more than 500 ml per 24 hrs). Low output fistulae often come from the colon or the distal small bowel and do not present such a clinical challenge as do high output fistulae which usually come from the proximal bowel, duodenum or stomach. The exact anatomy of an enterocutaneous fistula is usually delineated by a combination of clinical observation, biochemical analysis of fistula effluent and radiological investigation of which contrast studies, such as computed tomography (CT) and magnetic resonance imaging may influence the choice of treatment but radiological investigation is not always necessary in the early stages of management (Figures 1 and 2) [2].
Figure 1: Simple enterocutaneous fistula [45].
Figure 2: Complex enterocutaneous fistulae [45].
Fistula closure
Fistula closure depends on enterocutaneous fistula characteristics. Factors believed to predict spontaneous fistula closure are shown in Table 1. Multivariate analysis indicated that spontaneous closure was significantly more likely with low-output fistulas and significantly less likely when the fistula has a non-surgical (medical) aetiology, and when there were infective or non-infective complications [5,11]. Successful surgical closure was associated only with absence of infective and less with non-infective complications. Sepsis and inflammatory bowel disease can add to the patient’s state of malnutrition with a negative influence on fistula closure. Surgical technique was a predictor of fistula recurrence after surgery, with a 33% incidence of recurrence after wedge repair or over sewing, compared with 18% after resection of the fistula. This was even more pronounced in patients with Crohn’s disease (75 vs. 15%) [22]. Multivariable analysis of data from St Mark’s Hospital, London indicates successful closure associated with low initial fistula output and an absence of co-morbidity [3].
| Surgical Aetiology |
|---|
| Free distal flow |
| Healthy surrounding bowel |
| Simple fistula with no associated abscess cavity |
| Fistula tract >2cm |
| Fistula tract not epithelialised |
| Enteral defect <1cm ( with no discontinuity) |
| Low fistula output |
Table 1: Factors that predict spontaneous fistula closure.
Control of Fistula Output
The basic principles behind the reduction of fistula output are similar to those used in the management of short bowel syndrome [23,24]. The 24 hrs secretory function of the gut is illustrated in Table 2.
| Secretion | Amount(l) |
|---|---|
| Saliva | 1.5 |
| Gastric juice | 3 |
| Bile | 0.5 |
| Pancreatic juice | 1 |
| Intestinal juice | 3 |
| Total | 9 |
Table 2: 24 hrs secretory function of the gut.
With a high output fistula the fluid requirement may be in excess of 5 L per day. Paradoxically it will probably be necessary to restrict oral fluid intake because of the absence of sodium in the majority of drinks. Movement of sodium into the lumen occurs if sodium concentration is low and sodium absorption in the small bowel is actively linked to the absorption of glucose and certain amino acids. The intestinal response to these fluids of low sodium content is to secrete sodium and water into the lumen. In a normal person this extra fluid is readily re-absorbed lower in the ileum and colon. The patient with high output fistula does not have this opportunity and the losses of fluid and electrolytes readily become life-threatening. Fortunately the use of balanced electrolyte solutions as promoted for acute diarrhoeal illnesses overcomes this difficulty. The combination of oral solutions containing at least 60 mmol/l sodium, with restriction of other free (sodium lacking) fluids to no more than 500 ml/day will usually suffice in patients with more than 1 m of reasonably normal intestine above the fistula.
There is evidence that low-output fistulas have a greater rate of spontaneous closure than high output fistulas [11,25], but the evidence that reducing output increases the likelihood of spontaneous closure is less convincing. Thus, revealing the salient importance of other factors. Gastric secretions can be reduced using H2 receptor antagonists or proton pump inhibitors by 500 ml/24 hrs and bowel transit can be slowed with high doses of antimotility agents such as codeine phosphate or loperamide [24]. However, inhibitors of gastric acid secretion would not reduce fistula output enough to reduce the severity of intestinal failure in high output fistulas, and thus preventing the need for parenteral fluid and electrolyte replacement [23-25].
In adults, the antisecretory somatostatin analogue octreotide (50 μg s.c. b.i.d.) reduces fistula output with a reduction of volume of parenteral supplements [26,27]. Although some studies have shown no benefit as they do not increase the percentage of fistulae that close [28,29], there are good data that indicates that they may speed the closure [26,27]. A complication of the short bowel is the disruption of the ileocolonic brake mechanism mediated by hormones such as glucagon-like peptide 1 and 2 and peptide YY, which leads to gastric hyper secretion, rapid gastric and intestinal transit, and poor intestinal adaptation [30]. Clinical studies of their replacement with the peptide analogue teduglutide reduced parenteral fluid support [9,31] Therefore, the minimization of fluid and electrolyte fluxes aids in the management of accurate fluid and electrolyte balance, and may allow patient to be weaned off parenteral nutrition and fluid support (Table 3). In addition, reduction in the volume of irritant effluent facilitates skin care.
| Surgical Aetiology |
|---|
| Free distal flow |
| Healthy surrounding bowel |
| Simple fistula with no associated abscess cavity |
| Fistula tract >2cm |
| Fistula tract not epithelialised |
| Enteral defect <1cm ( with no discontinuity) |
| Low fistula output |
Table 3: Methods of reducing fistula output.
Malnutrition
Malnutrition is common and there are multiple factors that contribute to malnutrition in patients with enterocutaneous fistulas. The supply of nutrients may be limited, due to either anorexia or restriction of oral intake. Significant loss of protein, electrolytes and fluids can occur in fistula effluent as a result of loss of bowel secretions that would ordinarily be reabsorbed [24]. Patients with higher output fistulae will generally require parenteral support and sodium status is best monitored by urinary sodium measurement. Magnesium deficiency is also common in these patients and should be actively sought and treated as necessary [32,33]. The primary role of nutritional support, whether enteral or parenteral, is the prevention of malnutrition. Studies have shown a significantly higher incidence of complications and higher mortality rates in malnourished patients undergoing abdominal surgery for both benign and malignant gastrointestinal disease [6,34]. Evidence of an additional therapeutic role for TPN in the promotion of fistula healing is currently lacking. The concept of ‘bowel rest’ in this setting is based on the observation that gastrointestinal secretions dropped by 30-50% in patients receiving TPN [3,5] and that this might aid fistula closure. No randomized trials investigating patients kept exclusively ‘nil by mouth’ has been performed. Comparison could be drawn with data on the use of octreotide in decreasing fistula output but little evidence that it increases the chance of spontaneous fistula closure [26,29]. Thus Total Parenteral Nutrition (TPN) remain necessary in improving fluid and electrolyte balance in high output fistulas, but when the fistula is ileal or colonic, enteral (low residue) feeding will often allow sufficient bowel rest without the hazards of TPN (central venous catheter sepsis, venous thrombosis and pneumothorax), or loss of the protective effects that luminal nutrients have on the small bowel mucosa [3,35]. In addition, the necessary provision of parenteral nutrition in the hyper catabolic, septic patient with temporary intestinal failure should be judicious to avoid pulmonary edema and respiratory acidosis [36]. Although restriction of enteral intake and ‘bowel rest’ are often advocated, there is no evidence to suggest that such practice results in increased rate of fistula closure, and may even increase the incidence of complications [37]. Enteral feeding is not only safe but may improve anastomotic healing/ strength compared with parenteral feeding, possibly via trophic effects on the intestinal mucosa [38]. However, immune-enhancing diets such as glutamine supplementation in the critical care situation may actually worsen the systemic immune response syndrome and the precise role in this setting is controversial [39].
Principles of Management
Postoperative (Iatrogenic) Fistulas
The important steps for managing postoperative (iatrogenic) fistulas are:
Gaining fluid and electrolyte (i.e., metabolic) control. Stabilization should focus on correction of fluid depletion and any electrolyte imbalance. With high output enterocutaneous fistulas dehydration and hyponatraemia are common. There may be significant loss of potassium, chloride and bicarbonate ions, depending on the site of origin of the fistula, and the content of the effluent must be accounted for when calculating electrolyte replacement [5,40]. Blood transfusion may be required if there is significant anemia which may be due to the premorbid condition, postoperative, chronic blood loss, malnutrition or the bone-marrow depression of chronic sepsis, malignancy or chemo radiotherapy. As fluid balance can be difficult to assess even with a central line to monitor venous filling pressures, and a urinary catheter, daily weighing is highly desirable [12,24]. However, patients with generalized peritonitis from an anastomotic leak or perforated viscus cannot be fully resuscitated until ongoing soiling has been controlled. In such patients resuscitation should be continued intraoperatively (resuscitation surgery) with exteriorization of bowel ends as stomas [15,41] Laparostomy as opposed to primary closure of abdominal fascia may be indicated if there is a risk of developing an abdominal compartment syndrome from severe sepsis and septic shock [42].
Protecting the skin from the digestive effects of the enzymes and acid in the fistula effluent by expert application of stoma appliances and barrier products should be an early priority, especially with high output fistulas from the jejunum. The enzyme content of fistula effluent, coupled with prolonged exposure of the perifistula skin to moisture, can lead to rapid breakdown around a fistula. This prevents spontaneous closure and predisposes to infection. It is also painful and distressing to the patient. In addition, the collection of fistula effluent allows output to be measured accurately, thereby assisting fluid and electrolyte replacement [12,23].
Drain any sepsis as continuing sepsis is a potent cause for persistence of a fistula and for the patient to fail to improve clinically. Rapid and aggressive treatment is required as sepsis is the commonest cause of death [11,25,43,44]. Infection may be due to ongoing anastomotic leakage, to effects of the fistula effluent or to complications of treatment, such as central venous catheter infection, and it should be treated with appropriate antibiotics, guided by culture sensitivity. In addition, intra-abdominal collections should be drained externally under ultrasonographic or CT guidance, without undertaking a formal laparotomy. There are rare occasions in ill, septic patients where it is difficult to control the sepsis without recourse to early laparotomy and open drainage. In these circumstances it may be wise to raise a more proximal stoma and return later for definitive surgery. Occasionally exteriorization of the bowel ends will be required especially in septic patients with extensive abdominal contamination. Attempts at repair or anastomosis will be futile in these patients with significant malnutrition or sepsis [2].
Realize that the patient’s moral is paramount to recovery as most patients with an enterocutaneous fistula will be in hospital for a number of weeks or months. They have usually undergone major surgery and suffered a significant complication. The combination of an open wound and the production of fistula effluent is likely to have a detrimental effect on body image. If the fistula is not healing spontaneously they will be afraid of the often quite major surgery necessary to join the bowel ends together again. The patient’s morale is destroyed by failure to control the fistula but lifts when success has been achieved [12,23,24].
Wait as it will usually be six weeks before the patient is well enough and nutritionally repleted (i.e., in positive nitrogen balance) or the operative site has matured enough, for an attempt at curative surgery. Biological adhesiolysis is quite advanced by six weeks, so it seems wise to let nature do a lot of the surgeon’s work [2-7]. A fistula will generally close spontaneously by 6 weeks unless it: (a) originates from a diseased segment of bowel or continuing disease at the fistula site (e.g. Crohn’s, cancer, radiation damage), (b) arises from an anastomotic breakdown greater than 50% of the circumference of the bowel; (c) has a very short tract or communication between skin and mucosa (epitheliasation of the tract); (d) has bowel obstruction distal to it. If a fistula has not closed by 12 weeks, it probably never will [1-3,7,45].
Defining the anatomy of the fistula is important in planning management and predicting the long term outcome. Although it may be derived clinically, an earlier investigation may determine how much small bowel remains and the likely necessity for permanent parenteral nutrition [12]. CT scanning may also provide information on safe sites of entry into the abdominal cavity. This work-up includes radiological contrast studies including small bowel follow- through studies or enemas, and fistulograms to define the extent of intestinal disease, site of origin of the fistula, to exclude any obstructing lesions and delineate the anatomy of fistulous tracts. CT enterocyclis and Magnetic Resonance (MR) enterocyclis may demonstrate areas of ongoing pathology such as areas of ongoing obstruction and septic collections [46,47]. There are reports of vacuum-assisted dressings and gel foam embolization of the fistula tract achieving closure and facilitating enteral feeding. It is, however, not known whether the closure is simply accelerated or whether surgery can be avoided [48,49].
Operate later rather than earlier as early after surgery the tissues are friable and hold sutures poorly. Furthermore, at about 3 weeks from the original operation, adhesions are dense which makes surgery at this time dangerous. Delayed surgery also allows time for the metabolic and nutritional deficiencies to be corrected [2-5]. A definitive and curative operation can be done if timed right. The definitive operation entails complete enterolysis and the en bloc resection of the diseased or damaged bowel and the fistula tract with primary anastomosis. Any obstruction distally must be resolved, as must any area of continuing disease. A decision may sometimes have to be made whether to join the bowel ends together again or protect the fresh anastomosis with a temporary defunctioning stoma. On occasions the abdominal wound may be better left open initially and managed with a vacuum-assisted dressing, although there are some concerns that enterocutaneous fistulas can be induced in this way [12]. However, abdominal wall closure with biological mesh would maintain abdominal wall integrity and prevent enterocutaneous fistula formation [50,51].
Postoperative (Crohn’s) enterocutaneous fistula
Enterocutaneous fistulae in Crohn’s disease occur in the early postoperative period or at late stage because of anastomotic dehiscence or recurrent disease. Rarely a late fistula may result from residual sepsis associated with a previously contained anastomotic leak. With an early postoperative fistula, the fecal stream should be externally diverted proximal to the leak and this may be coupled with resection of the fistula and bowel anastomosis if peritoneal reaction is minimal. For later appearing septic fistulae more than 10 postoperative days), re-entry into the peritoneal cavity can be dangerous because of dense adhesions and the risk of multiple enterotomies [52]. In this instance, parenteral nutrition and sepsis management usually provide an acceptable outcome. Late enterocutaneous fistulae due to recurrent disease will usually require resection and anastomosis, when feasible [53]. Very low output fistulae may be managed by non- operative techniques in patients whose operative risk is significant. In such instances, the immunosuppressives, (azathioprine, cyclosporine) and immunomodulatory Tumour Necrosis Factor (TNF) α (infliximab) have been valuable in augmenting fistula closure [54].
Spontaneous enterocutaneous fistula
Although, spontaneous and postoperative (iatrogenic) fistulas behave differently, the same management principles would apply [2,21]. The main difference is that after the initial stabilization of the patient following resuscitation, it is reasonable to anticipate spontaneous closure of iatrogenic fistulas with conservative measures by 6 weeks but not spontaneous fistulas as the diseased segment of the bowel will not heal spontaneously and require resection. In addition, the spontaneous enterocutaneous fistulas will generally benefit from earlier surgery. There is no concern about a more recent laparotomy making surgery difficult. The bowel perforation occurs slowly and abdominal sepsis is usually localized, lessening the initial systemic insult. In addition, although the aim is to optimize the patient’s general and nutritional state before surgery, active Crohn’s disease will limit what is achievable [55-57]. In expert hands surgical repair is successful in closing the fistula in more than 95% of cases although failure after an attempt at definitive surgery can result in a high mortality rate [2,15,57,58]. Best results are derived from operation within 24 hrs with resection of the diseased segment and exteriorization of the bowel ends [20].
Conclusions
The immediate management of the patient with an enterocutaneous fistula follows resuscitation which entails the replacement of fluid and electrolyte losses and the control of sepsis by adequate drainage of abscesses. The management is heavily influenced by the underlying aetiology (spontaneous or iatrogenic) and the anatomical classification (simple fistula or complex fistula). Together these will determine the likelihood of resolution with or without surgical intervention. The goal is for fistula closure with minimum morbidity or mortality. Patients with enterocutaneous fistula can now almost always be cured, if the right steps are adopted.
Ethical Approval
No ethical approval was required as it is a simple review article.
Funding
A quarterly research allowance to university lecturers from the Ministry of Higher Education, Cameroon.
Author Contribution
Mr. Elroy Patrick Weledji is the sole author.
Conflict of Interest
The author has no conflict of interest.
References
- Altomare DF, Serio G, Pannarale OC, Lupo L, Palasciano N, et al. (1990) Prediction of mortality by logistic regression analysis in patients with postoperative enterocutaneous fistulae. Br J Surg 77: 450-453.
- Rolandelli R, Roslyn JJ (1996) Surgical management and treatment of sepsis associated with gastrointestinal fistulas. Surg Clin North Am 76: 1111-1122.
- Lloyd DAJ, Gabe SM, Windsor ACJ (2006) Nutrition and management of enterocutaneous fistula. Br J Surg 93: 1045-1055.
- Windsor JA, Hill GL (1988) Protein depletion and surgical risk. Aust N Z J Surg 58: 711-715.
- Gonzalez-Pinto I, Gonzalez EM (2001) Optimising the treatment of upper gastrointestinal fistulae. Gut 49 (Suppl 4).
- Meguid MM, Campos AC (1996) Nutritional management of patients with gastrointestinal fistulas. Surg Clin North Am 76: 1035-1080.
- Berry SM, Fischer JE (1996) Classification and pathophysiology of enterocutaneous fistulas. Surg Clin North Am 76: 1009-1018.
- Pearse RM, Morenon RP, Bauer P, Pelosi P, Metnitz P, et al. (2012) Mortality after surgery in Europe: a 7 day cohort study. Lancet.
- Meguid MM, Debonis D, Meguid V, Hill LR, Terz JJ (1988) Complications of abdominal operations for malignant disease. Am J Surg 156: 341-345.
- Leslie A, Steele RJC (2003) The interrupted serosubmucosal anastomosis- still the gold standard. Colorectal Dis 5: 362-366.
- Campos AC, Andrade DF, Campos GM, Matias JE, Coelho JC (1999) A multivariate model to determine prognostic factors in gastrointestinal fistulas. J Am CollSurg 188: 483-490.
- Vaizey C, Warusavitarne J (2009) Intestinal failure In: Phillips Robin, editor. A companion to specialist surgical practice- colorectal surgery. (4thedn.) Saunders, Elsevier, 41.
- Moran BJ, Heald RJ (2001) Risk factors for, and management of, anastomotic leakage in rectal surgery. Colorectal Dis 3: 135-137.
- Krukowski ZH, Matheson NA (1988) A ten year computerized audit of infection after abdominal surgery. Br J Surg 75: 857-861.
- Weledji EP, Ngowe NM (2013) The challenge of intraabdominal sepsis. Int J Surg 11: 290-295.
- Jr Edmunds LH, Williams GM, Welch CE (1960) External fistulas arising from the gastrointestinal tract. Ann surg 152: 445-471.
- West JP, Ring EM, Miller RE, Burks WP (1961) A study of the causes and treatment of external postoperative intestinal fistulas. SurgGynecolObstet 113: 490-496.
- Galland RB, Spencer J (1986) Radiation-induced gastrointestinal fistulae. Ann R CollSurgEngl 68: 5-7.
- Roy D (1986) Surgery in tropical countries. In: Taylor, O. Higgin, Chisholm, editors. Surgical management. London: William Heinemann Medical Books Ltd.
- Travis SP, Stange EF, Lemann M (2006) European evidence based consensus on the diagnosis and management of Crohn’s disease: current management. Gut 55: 16-35.
- Fischer JE (1983) The pathophysiology of enterocutaneous fistulas. World J Surg 7: 446-450.
- Lynch AC, Delaney CP, Senagore AJ, Connor JT, Remzi FH, et al. (2004) Clinical outcome and factors predictive of recurrence after enterocutaneous fistula surgery. Ann Surg 240: 825-831
- Nightingale J, Woodward J (2006) Guidelines for management of patients with a short bowel. Gut 55 (Suppl. IV-1-12).
- Nightingale JM (2003) The medical management of intestinal failure: methods to reduce the severity. ProcNutrSoc 62: 703-710.
- Haffejee AA (2004) Surgical management of high output enterocutaneous fistula e: a 24-year experience. Curropin Clin NutrMetab Care 7: 309-16.
- Hesse U, Ysebaert D, de Hemptinne B (2001) Role of Somatostatin-14 and its analogues in the management of gastrointestinal fistulae: clinical data. Gut 49: 21.
- Alvizatos V, Felekis D, Zorbalas A (2002) Evaluation of the effectiveness of octreotide in the conservative treatment of postoperative enterocutaneous fistulas. Hepatogastroenterology 49:1010-1012.
- Scott NA, Finnegan S, Irving MH (1993) Octreotide and postoperative enterocutaneous fistulae: a controlled prospective study. ActaGastroenterolBelg 56: 266-270.
- Alvarez C, McFadden DW, Reber HA (2000) Complicated enterocutaneous fistulas: failure of octreotide to improve healing. World J Surg 24: 533-537.
- Weledji EP (2016) Complications of non-occlusive mesenteric ischaemia. Acute MedSurg 3: 50-52.
- Jeppesen PB, Pertkiewicz M, Messing B (2012) Teglutide reduces need for parenteral support among patients with short bowel syndrome with intestinal failure. Gastroenterology 143: 1473-1481.
- Dardai F, Stefanics J (1983) Total parenteral nutrition in management of external small bowel fistulae. ActaChir Hung 24: 97-103.
- Dardai F, Pirityl S, Nagy L (1991) Parenteral and enteral nutrition and the enterocutaneous fistula treatment. II. Factors influencing the outcome of treatment. ActaChir Hung 32: 305-318.
- Mughal MM, Meguid MM (1982) The effect of nutritional status on morbidity after elective surgery for benign gastrointestinal disease. JPEN J Parenter Enteral Nutr 11: 140-143.
- Alpers DH (2002) Enteral feeding and gut atrophy. CurrOpin Clin NutrMetab care 5: 679-683.
- Mclauchan GJ, Anderson ID, Grant IS, Fearon KCH (1995) Outcome of patients with abdominal sepsis treated in an intensive care unit. Br J Surg 82: 624-629.
- Lewis SJ, Egger M, Sylvester PA, Thomas S(2001) Early enteral feeding versus ‘nil by mouth’ after gastrointestinal surgery : systematic review and meta-analysis of controlled trials BMJ 323: 773-776.
- Kiyama T, Efron DT, Tantry U, Barbul A (1999) Effect of nutritional route on colonic anastomotic healing in the rat. J. GastrointestSurg 3441-3446.
- Bertolini G, Iapichino G, Radrizzani D, Facchini R, Simini B, et al. (2003) Early enteral immunonutrition in patients with severe sepsis : results of an interim analysis of a randomized multicentre clinical trial. Intensive care Med 29: 834-840.
- Foster CE III, Lefor AT (1996) General management of gastrointestinal fistulas. Recognition, stabilization and correction of fluid and electrolyte imbalances. Surg Clin North Am 76: 1019-1033.
- Waibel BH, Rotondo MF (2012) Damage control for intra-abdominal sepsis. Surg Clin North Am 92: 243-247.
- Schein M (2003) Planned relaparotomies and laparostomy. In: Schein M, Marshall JC, editors. A guide to the management of surgical infections. Heideberg-Springer. pp: 412-423.
- Hollington P, Mawdsley J, Lin W, Gabe SM, Forbes A, et al. (2004) An 11-year experience of enterocutaneous fistula. Br J Surg 91: 1646-1651.
- Reber HA, Roberts C, Way LW, Dunohy JF (1978) Management of external gastrointestinal fistulas. Ann Surg 188: 460-477.
- Weledji EP, Chichom AM (2012) Aetiology and impact of intra-abdominal sepsis on surgical management. East Cent Afr J Surg 17:3.
- Lappas JC (2003) Imaging of the postsurgical small bowel. Radiol Clin North Am 41: 305-326.
- Wiarda BM, Kuipers EJ, Houdijk LP (2005) MR enterocyclis: imaging technique of choice in diagnosis of small bowel diseases. Dig Dis Sci 50: 1036-1040.
- Hyon SH, Martinez-Garbino JA, Benati ML, Lopez-Avellaneda ME, Brozzi NA, et al. (2000) Management of a high-output postoperative enterocutaneous fistula with a vacuum-sealing method and continuous enteral nutrition. ASAIO J 46: 511-514.
- Medioros AC, Aires-Neto T, Marchini JS, Brandao-Neto J, Valenca DM, et al. (2004) Treatment of postoperative enterocutaneous fistulas by high- pressure vacuum with a normal oral diet. Dig Surg 21: 401-405.
- Adkins AL, Robbins J, Villalba (2004) Open abdomen management of intra-abdominal sepsis. Am Surg 70: 137-140.
- Schein M, Decker GA (1990) Gastrointestinal fistulas associated with large abdominal wall defects: experience with 43 patients. Br J Surg 77: 97-100.
- Bernell O, Lapidus A, Hellers G (2000) Risk factors for surgery and postoperative recurrence in Crohn’s disease. Ann Surg231: 38-45.
- Fischera A, Lovadina S, Rubin M (2006) Patterns and operative treatment of recurrent Crohn’s disease: a prospective longitudinal study. Surgery 140: 649-654.
- Sands BE, Anderson FH, Bernstein CN (2004) Infliximab maintenance therapy for fistulizing Crohn’s disease. N Engl J Med 350: 876-885.
- Li J, Ren J, Zhu W, Yin L, Han J (2003) Management of enterocutaneous fistulas: 30- year clinical xperience. Chin Med J (Engl) 116: 171-175.
- McIntyre PB, Ritchie JK, Hawley PR, Bartram CI, Leonard-Jones JF (1984) Management of enterocutaneous fistulas: a review of 132 cases. Br J Surg 71: 293-296.
- Martinez D, Zibari G, Aultman D, McMillan R, Mancini MC, et al. (1998) The outcome of intestinal fistulae : the Loisisiana State University Medical center-Shreveport experience. Ann Surg 64: 252.
- Weledji EP, Puepi MA, Chichom AM (2014) A rare spontaneous enterocutaneous fistula. J Surg Case Rep pp: 1-3.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences