Role of Q-Waves ECG in Myocardial Scar Assessment in patients with Prior Myocardial Infarction
Lucia PV, Anna LL, Catherine K, Tiziano M and Francesco FF
DOI10.36648/2471-299X.5.2.79
Lucia PV1*, Anna LL1, Catherine K1, Tiziano M1 and Francesco FF2
1Fondazione Cardiocentro Ticino, Lugano, Switzerland
2Clinical Epidemiology & Biometry, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy
- *Corresponding Author:
- Paiocchi Vera Lucia
Fondazione Cardiocentro Ticino, Lugano
Switzerland
E-mail:paiocchivera@gmail.com
Received date: May 16, 2019 Accepted date: June 21, 2019 Published date: June 28, 2019
Citation: Lucia PV, Anna LL, Catherine K, Tiziano M, Francesco FF (2019) Role of Q-Waves ECG in Myocardial Scar Assessment in Patients with Prior Myocardial Infarction. Med Clin Rev Vol. 5 No. 2: 4.
Copyright: © 2019 Lucia PV, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Keywords
Ischemic heart disease; Electrocardiogram; Qwaves; Transmural scar
Abstract
This study was designed to assess the sensitivity and specificity of the pathological Q waves as defined in Electrocardiogram (ECG) criteria of European Society Guidelines (ESC) in myocardial scar assessment in patients with prior myocardial infarction. In common clinical practice, Q waves, or QS complexes in the absence of QRS confounders are pathognomonic of prior Myocardial Infarction (MI) in patients with chronic Ischemic Heart Disease (IHD) regardless of symptoms. Prior MI is characterized by the presence of scar. Cardiac Magnetic Resonance (cMRI) Late Gadolinium Enhancement (LGE) is considered the gold standard technique for the detection of myocardial scar. Data was collected on 500 patients referred for a 3 Tesla cMRI viability study. A 12-ECG lead was recorded in each patient. Sensitivity and specificity of wallspecific ECG changes in presence of 2+ or 3+ pathological Q waves in the corresponding wall leads have been evaluated for anterior (V1-V4 leads), inferior (D2, DIII, aVF leads) and lateral (D1, aVL, V5-V6 leads) wall in patients with transmural infarction, defined as >50% LGE. The sensitivity and specificity of wall-specific ECG changes in presence of 2+ pathological Q-waves were 42% and 88% for anterior, 43% and 69.9% for inferior and 28.6% and 76% for lateral wall; in presence of 3+ Q waves they were 24% and 95% for anterior, 27.8% and 82.5% for inferior and 9.5% and 93.8% for lateral wall. This study suggests that Q waves ESC ECG criteria may be a poor marker for detecting myocardial scar in patients with prior MI.
Introduction
Nowadays the Electrocardiogram represents the easiest medical instrument approach for assessing several heart clinical conditions such as ischemic heart disease. Indeed, the usefulness of the electrocardiogram in clinical practice is well known, due to the fact that ECG is a very simple non-invasive technique, easily available and economic. In common clinical practice, we know that Q waves or QS complexes, in the absence of QRS confounders, are pathognomonic of a prior myocardial infarction in patients with chronic ischemic heart disease regardless of symptoms [1]. Prior myocardial infarction is characterized by the presence of myocardial scar. In the past, studies about the correlation between electrocardiographic abnormalities and the presence of myocardial scar has been severely limited in literature and involved small cohorts of patients. This is because such studies have employed necropsy or angiographic observations as standards of reference against which to assess the properties of the ECG criteria [2-4]. Nevertheless in recent years, these limitations have been largely overcome by the increasing use of advanced imaging techniques such as Cardiac Magnetic Resonance Late Gadolinium Enhancement which rapidly has become the gold-standard technique for in-vivo identification of myocardial scar [5,6]. So inevitably, in the current era of new imaging technique the “Qwave” first described that it [7] is increasingly challenged by the direct visualization of scar using delayed enhancement magnetic resonance.
In literature many studies defend the role of Q-wave, citing its specificity for post-infarct scar [8], ability to localize the site of injury [9], and identify large infarcts among other attributes [10-12]. The aim of this study is to assess the sensitivity and the specificity of the current ECG criteria as described in Third definition of myocardial infarction (ESC Guidelines 2012) in identifying the presence and location of prior MI as detected by cMRI. In particular the sensitivity and specificity of wall-specific ECG changes considering the presence of 2+ or 3+ pathological Q waves in the corresponding wall leads have been evaluated for anterior, inferior and lateral walls in patients with transmural infarction, defined as >50% LGE.
Methods
Study population
This was a retrospective single-center study consisted of 500 patients (mean age 66.1 ± 14.1 years; 110 females and 390 males) who were referred for a 3 Tesla cMRI for evaluation of myocardial viability and necrosis at our institution. A 12-ECG lead was performed before cMRI scan. cMRI scan were performed in stable patient during hospital admission. Exclusion criteria of the study: claustrophobic patients, presence of cMRI un-safety devices, arrhythmic disorders (atrial fibrillation, frequent supra-ventricular and ventricular ectopies, advanced atrio-ventricular conduction disorders), and severe renal function impairment (patients with eGFR<30 mL/min/kg).
Electrocardiogram
A 12-lead ECG was recorded in each patient enrolled in the study before cMRI scan. Right and posterior leads were not analyzed. Rhythm, heart rate, duration and amplitude of all waves (P, Q, R, S, R’, S’, T) were analyzed manually by two experienced observers. The Q, S, and S’ waves were defined as the first, the second and the third negative deflection between P and T interval. According to this definition, a QS complex was recorded as a single Q wave of period equal to the QS complex in the absence of R-waves, S, R’ and S’. Pathologic Q-waves were defined according to ECG-Q wave’s criteria of the Third Universal definition of myocardial infarction as following:
-Any Q waves in leads V2-V3>/= 0.02 sec or QS complex in leads V2-V3.
-Q wave>/= 0.03 sec and >/=0.1 mV deep or QS complex in leads I, II, aVL, aVF, or V4-V6 in any two leads of a contiguous lead grouping (I, aVL; V1-V6; II, III, aVF).
-R wave>/= 0.04 sec in V1-V2 and R/S>/=1 with concordant positive T wave in absence of conduction defect.
The presence of 2+ or 3+ pathological Q waves in the corresponding wall leads for anterior, inferior, and lateral walls were analysed: anterior wall explored by V1-V2-V3-V4 leads, inferior wall by D2-D3-aVF and lateral wall by D1-aVL and V5 -V6 leads.
Late gadolinium- enhanced cardiac magnetic resonance
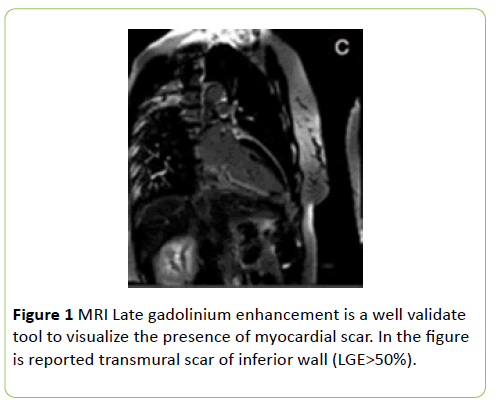
All patients were examined in the supine position using a 3 Tesla scanner (Magnetom Skyra; Siemens Healthcare, Erlangen, Germany) with a 32-channel phased-array coil while holding their breath. After localization, cine sequences were acquired using balanced Steady-State Free Precession sequences (SFFP) with long (2-3-4 chambers) and short axis images. Short axis acquisitions were performed using multiple slices to cover both ventricles entirely from the base to the apex (field of view, 350x295; matrix size, 216x256; acceleration factor, 2 generalized auto-calibrating partial parallel acquisition; slice thickness, 8 mm; interslice gap, 20%, number of phases, 25). To evaluate myocardial scars, late enhancement acquisition (2D phasesensitive segmented, inversion recovery gradient echo) were performed 10 minutes after an intravenous bolus injection of 0.2 mmol/kg gadolinium-based contrast. Positive hyper enhancement was visually defined as >2 SDs of the signal intensity of the non-enhanced myocardium. Short and long-axis slices (8 mm) were acquired on a prospective electrocardiographically gated gradient-echo sequence with inversion pre-pulse using the same slices of cine sequences (field of view 330X 330; matrix size ,192X 256; acceleration factor, 2 generalized auto-calibrating partial parallel acquisitions; slice thickness, 8 mm; interslice gap, 20%; inversion time set according to scout images). Biventricular volumes, function, and left ventricular mass were measured using a standard volumetric technique from the cine short axis images. Ventricular volumes and mass measurements were indexed to body surface area. Left ventricular segmental function using the 17-segment cardiac model [13] was quantified with the following numerical score: 0=normal kinetic, 1=hypokinesia, 2=akinesia, 3=dyskinesia. Each myocardial segment was evaluated for the presence of different percentage of late gadolinium enhancement: normal myocardium LGE=0%; subendocardial infarction LGE>25%; transmural infarction as LGE>50% (Figure 1).
Statistical analysis
Computations were performed using STATA version 14.1 (StataCorp, College Station, TX). Data are expressed as mean ± SD if continuous and as counts and percentages if categorical. The analyses were performed by using logistic regression with dummy variables used to assess scar presence. The following analyses were carried out: 1) Correlation between pathological Q waves (2+ or 3+) and presence of transmural scar (LGE>50%); 2) Correlation between pathological Q waves (2+ or 3+) in the corresponding wall leads and transmural scar (LGE>50%) for anterior, inferior, lateral wall 3) Correlation between pathological Q waves (2+ or 3+) and presence of scar at different percentage of LGE (0%-25%-50%).
Results
Patients characteristics
The study population consisted of 500 patients, (mean age 66.1 ± 14.1 years; 110 females (22%) and 390 males (78%); 183 healthy control subjects; 303 had hypertension (62%), 274 dyslipidemia (58%), 147 had a family history of cardiovascular heart disease (30%), 134 smokers (28%), 95 with diabetes (19%). The baseline characteristic of study population is described in Table 1. The population study includes more males than women (78% vs 22%). The study population is characterized by the most frequent cardiovascular risk factor: hypertension (62%), dyslipidemia (58%), BMI>25 (overweight).
Table 1 Baseline characteristic of study population.
| Variable | Value |
|---|---|
| Age (years) | 66.1 ± 14.1 |
| (n) Male (%) | 390 (78%) |
| (n) Female (%) | 110 (22%) |
| Weight (kg) | 79.9 ± 15.9 |
| Height (cm) | 170.5 ± 8.5 |
| BMI (kg/m2) | 27.3 ± 4.4 |
| BSA (m2) | 1.9 ± 0.2 |
| Hypertension | 303 (62%) |
| Dyslipidemia | 274 (58%) |
| Diabetes | 95 (19%) |
| Smoke | 134 (28%) |
| History of CAD | 147 (30%) |
Electrocardiogram and cardiac magnetic resonance
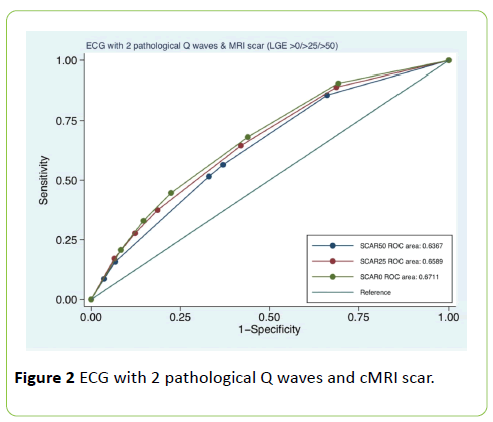
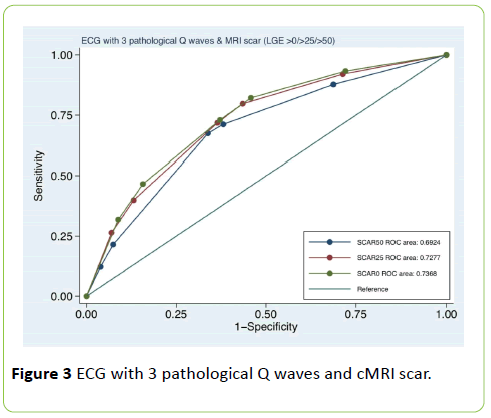
All patients were in sinus rhythm at the time of the examination with mean heart rate of 67.8 ± 12.4 beats/min. The mean duration of PR interval was 172.4 ± 31.6 sec; the mean duration of QRS interval 106.3 ± 23.6 sec; the mean duration of QT interval 406.9 ± 39.2 sec and QT corrected interval (QTc) 428.6 ± 36.1 sec. Left ventricle: mean EDV 87.6 ± 32.4 mL/m2, ESV 43.9 ± 30.5 mL/m2, SV 43.7 mL/m2 ± 10.1; mean ejection fraction 0.53% ± 0.13, LV mass 68.6 ± 18.2 g/m2. Right ventricle: mean EDV was 53.7 ± 36.9 mL/m2 and mean ejection fraction 0.58% ± 0.09. The characteristic of electrocardiogram and cMRI are described in Table 2. In Table 3 the number (percentage) of pathological Q waves (2+ -3+) for each ventricular site (anterior, inferior, and lateral wall) are reported. For anterior wall pathological Q waves 2+ were 78 (15%) and Q waves 3+ 35 (7%); for inferior wall 160 (32%) and 95 (19%), for lateral wall 123 (24%) and 33 (6.6%) respectively. In Table 4 the agreement of pathological Q-waves (2+ or 3+) and transmural scar (LGE>50%) is described. In presence of pathological Q waves (2+) the sensitivity and specificity were 38.1% and 78.1% respectively, ROC area 0.58, kappa 0.11, Std. Error 0.02; in presence of 3+ pathological Q waves the sensitivity and specificity were 20.8% and 90.6%, ROC area 0.55, Kappa 0.12, SE 0.02 (interpretation of kappa values: below 0.0=poor; 0.0 to 0.20 slight; 0.21 to 0.40 fair; 0.41 to 0.60 moderate; 0.61 to 0.80 substantial; 0.81 to 1.00 almost perfect). In Table 5 the association of pathological Q waves and scar is reported for each ventricular wall (anterior, inferior and lateral). The sensitivity and specificity in presence of 2+ pathological Q-waves were 42% and 88% for anterior wall, 43% and 69.9% for inferior wall and 28.6% and 76% for lateral wall; AUC ROC 0.65, 0.56 and 0.52 respectively; in presence of 3+ pathological Q-waves they were 24% and 95% for anterior wall, 27.8% and 82.5% for inferior wall and 9.5% and 93.8% for lateral wall; AUC ROC 0.59; 0.55; 0.51. In Figures 2 and 3 ROC area curve of pathological Q waves (2+ or 3+) and presence of myocardial scar at different percentage of LGE (0%>25%>50%) are described. In presence of 2+ pathological Q-waves the ROC area values were 0.63 for LGE>50%; 0.65 for scar>25%; 0.67 for scar >0%; in presence of 3+ pathological Q-waves the ROC area values were 0.69; 0.72 and 0.73 respectively.
Table 2 ECG and cMRI parameters.
| Variable | Value |
|---|---|
| Heart rate (beats/min) | 67.8 ± 12.4 |
| PR interval (sec) | 172.4 ± 31.6 |
| QRS interval (sec) | 106.3 ± 23.6 |
| QT interval (sec) | 406.9 ± 39.2 |
| QTc interval (sec) | 428.6 ± 36.1 |
| LV EDV (mL/m2) | 87.6 ± 32.4 |
| LV ESV (mL/m2) | 43.9 ± 30.5 |
| LV SV (mL/m2) | 43.7 ± 10.1 |
| LV EF (%) | 0.53 (%) ± 0.13 |
| LV mass (g/m2) | 68.6 ± 18.2 |
| RV EDV (mL/m2) | 53.7 ± 36.9 |
| RV EF (%) | 0.58 (%) ± 0.09 |
Table 3 ECG and cMRI parameters.
| Site | Q2+ | Q3+ |
|---|---|---|
| Anterior | 78 (15%) | 35 (7%) |
| Inferior | 160 (32%) | 95 (19%) |
| Lateral | 123 (24%) | 33 (6.6%) |
Table 4 Site of Q2+ and Q3+ waves.
| Variable | Sensitivity | Specificity | Std.Err. | Odds Ratio | Kappa | AUC ROC |
|---|---|---|---|---|---|---|
| 2 + Q-waves | 38.10% | 78.10% | 0.02 | 2.17 | 0.11 | 0.58 |
| 3+ Q-waves | 20.80% | 90.60% | 0.02 | 2.52 | 0.12 | 0.58 |
Table 5 Sensitivity and Specificity of Q waves.
| Q-waves 2+ | Sensitivity | Specificity | Std.Err | Odds Ratio | Kappa | AUC ROC |
|---|---|---|---|---|---|---|
| Anterior (V1-V4) | 42% | 88% | 0.04% | 5.36 | 0.27 | 0.65 |
| Inferior (II-III-aVF) | 43% | 69.90% | 0.03 | 1.75% | 0.08 | 0.56 |
| Lateral (I-aVL-V5-V6) | 28.60% | 76% | 0.04 | 1.26% | 0.03% | 52.00% |
| Q-waves 3+ | Sensitivity | Specificity | Std.Err | Odds Ratio | Kappa | AUC ROC |
| Anterior (V1-V4) | 24.20% | 95.40% | 0.04 | 6.67 | 0.24 | 0.59 |
| Inferior (II-III-aVF) | 27.80% | 82.50% | 0.04 | 1.81% | 0.09 | 0.55 |
| Lateral (I-aVL-V5-V6) | 9.52% | 93.80% | 0.04 | 1.60% | 0.04 | 0.51 |
Discussion
Ischemic heart disease is a major cause of death and disability in developed countries [14]. Although the mortality for this condition has gradually declined over the last decades in western countries, it still causes about one-third of all deaths in people older than 35 years [15,16]. Early diagnosis of ischemic heart disease is crucial, because of the life-threatening consequences of this pathology. The diagnosis is mainly based on some risk stratification approaches, including medical history, physical examination, cardiac serum biomarkers, and instrumental approaches as electrocardiogram. In daily clinical practice the electrocardiogram is uniformly considered one of the first initial cardiovascular testing and an important routine part for the assessment of this category of patients. We know also that invasive approaches, as coronary angiography, is a useful medical tool for detecting patients with suspected CAD, especially in patients with a high pre-test probability of coronary arteries disease, but sometimes characterized by a high risk of procedure complications. On these assumptions, the use of a non-invasive assessment tool for detecting ischemic heart disease has been recently considered largely because offers safety and faster performance, with low risk of adverse effects. So, the electrocardiogram remains undoubtedly a cornerstone in the management of cardiovascular disease, but its value has been challenged in some conditions by newer diagnostic and imaging modalities such as cardiac magnetic resonance and in particularly in diagnosing of prior myocardial injury. In the past years, many studies focused on the predictive value of the ECG in assessing myocardial scar, but characterized by a few number of patients enrolled [8,10].
The study of Asch et al. was a single-centre study of 146 patients referred for CMR for evaluation of myocardial viability and necrosis. Q/QS waves on ECG were defined as per Minnesota Code criteria. Myocardial scar was quantified and localized by CMR delayed contrast hyper-enhancement and assumed as criterion standard. Sensitivity, specificity, and predictive values of ECG were calculated for different scar sizes (>1%, >15%, and >30% of the myocardium) and location (global, anterior, inferior, and lateral walls). In this study the lack of sensitivity and the resulting low negative predictive value of Q/QS criteria seriously limit its accuracy as a marker of prior MI. In a study [8] it was investigated how pathologic Q waves or equivalents predict location, size, and transmural extent of Myocardial Infarction (MI). A group of 79 consecutive patients with a previous first Q-wave MI, documented by clinical records, was studied prospectively by contrast-enhanced MRI and with ECG. Their conclusion was that the location of Q waves in specific ECG leads can reliably predict MI location, size, and transmural extent only in patients with anterior MI and a tall and broad R wave in V1-V2 reflects a lateral (not a posterior) MI. Our study consisted of 500 patients, and in literature is the study with the highest number of patient enrolled. Our study focused on the accuracy of pathological Q-waves in detecting prior myocardial infarction. For this purpose, the current ECG criteria (ESC guidelines 2012) were analysed to assess the presence and location of transmural scar (LGE>50%). CMR LGE is considered the gold standard technique to assess the presence and location of myocardial scar (Figure 1), however have a number of limitations, including high cost, accessibility and a limited number of medical experts trained in. Therefore, the purpose of our study is to validate the use of Q-waves ECG in routine clinical practice in the absence to access to other modalities. In this study we analysed firstly the agreement of pathological Q waves (2+ or 3+ per leads) and the presence of transmural myocardial scar (LGE>50%). The ability of the actual Q-waves ECG criteria in identifying the presence of myocardial scar is poor for the modest value of the ROC curve and for the sensitivity and specificity values (Table 4). Looking at our results, the ECG accuracy in detecting the site of myocardial scar its better in presence of 3+ pathological Q waves per leads instead of 2+. Another interesting result that we can deduce from our analyses is that the ability of Q waves ECG criteria in detecting myocardial scar depends on the site of scar. ROC curve values in presence of 2+ pathological Q waves were: 0.65 for anterior -0.56 inferior, 0.52 lateral wall; sensitivity: 42%, 43%, 28.6% and specificity: 88%, 69.9%, 76% respectively; ROC curve values considering the presence of 3+ pathological Q waves were: 0.59, 0.55, 0.51; sensitivity values for each wall 24.2%, 27.8%, 9.52% and specificity: 95.4%, 82.5%, 93.8% respectively. Looking these results, the ability of the electrocardiogram to discriminate is still modest as we have seen previously in literature. Lastly we considered the ability of the electrocardiogram in detecting myocardial scar considering different amount percentage of LGE (>0%>25%>50%) in CMR. Also in this case, the ability of electrocardiogram to discriminate the presence of myocardial scar is poor with low values of the ROC curves, as showed in Figures 2 and 3. Looking to our results, the ECG is a poor marker to assess prior myocardial injury. In order to improve the ability of the Q-wave to identify myocardial scar, it is necessary to reexamine its pathological basis.
Although Q waves are considered to represent depolarizing current from opposing myocardial walls though “a window” of infarcted tissue [17], studies at autopsy and LGE-CMR now show that scar is rarely homogeneous and often exhibits strands of interspersed viable tissue [18]. The signal-averaged ECG was initially developed to indicate heterogeneous conduction through heterogeneous scar yet, though predictive for arrhythmic events in patients with reduced left ventricular ejection fraction post-MI [19], but its accuracy for determining scar burden is undefined [20]. Das et al. [21] have described the index of fragmented QRS (fQRS), defined as the presence of R’ or notching in the nadir of the S-wave (QRS<120 ms) in 2 contiguous leads corresponding to a major coronary artery territory, which may represent conduction through islands of viable tissue within scar. Recent studies show that the fQRS adds to the predictive value of Q waves for myocardial scar, although the Q-wave has higher specificity and positive predictive value for scar and prior MI than fQRS. Therefore this is a potentially exciting area for future work.
Conclusion
In this study, we analyse the role of the current ESC ECG criteria to assess the presence and location of myocardial scar in patients with chronic ischemic heart disease. This study suggests the low accuracy of the ESC ECG 2012 criteria for the assessment of prior myocardial infarction, in particular for the detection and location of myocardial scar. Clinicians need to be aware of the limitations of the ECG instrument in evaluation of patients with chronic ischemic heart disease. Based on achieved results a redefinition of the actual ECG criteria for the assessment of prior myocardial infarction should be assessed. In conclusion cCMR is to be considered currently the best imaging technique in detecting myocardial scar, its severity and its location and, where is possible, its use is strongly recommended in clinical practice.
References
- Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, et al. (2012) The writing group on behalf of the joint esc/accf/aha/whf task force for the universal definition of myocardial infarction. Third universal definition of myocardial infarction. Eur Heart J 33: 2551-2567.
- Savage RM, Wagner GS, Ideker RE, Podolsky SA, Hackel DB (1977) Correlation of post-mortem anatomic findings with electrocardiographic changes in patients with myocardial infarction: retrospective study of patients with typical anterior and posterior infarcts. Circulation 55: 279-285.
- Horan LG, Flowers NC, Johnson JC (1971) Significance of the diagnostic Q wave of myocardial infarction. Circulation 43: 428-436.
- Uusitupa M, Pyorala K, Raunio H, Rissanen V, Lampainen E (1983) Sensitivity and specificity of the minnesota code Q-QS abnormalities in the diagnosis of myocardial infarction verified at autopsy. Am Heart J 106: 753-757.
- Lin D, Kramer CM (2008) Late gadolinium–enhanced Cardiac Magnetic Resonance. Current Cardiology Reports 10: 72-78.
- Kim RJ, Timothy SE, James H, Michael D, John CA, et al. (2008) Myocardial infarction imaging investigators performance of delayed enhancement magnetic resonance imaging with gadoversetamide contrast for the detection and assessment of myocardial infarction an international, multicenter, double-blinded, randomized trial. Circulation 117: 629-663.
- Pardee HEB (1930) The significance of an electrocardiogram with a large Q in lead 3. Arch Intern Med 46: 470-481.
- Asch FM, Shah S, Rattin C, Swami-nathan S, Fuisz A, et al. (2006) Lack of sensitivity of the electrocardiogram for detection of old myocardial infarction: a cardiac magnetic resonance imaging study. Am Heart J 152: 742-748.
- De Luna AB (2006) Location of Q-wave myocardial infarction in the era of cardiac magnetic resonance imaging techniques. J Electrocardiol 39: S79-81.
- Rovai D, Di Bella G, Rossi G, Lombardi M, Aquaro GD, et al. (2007) Q-wave prediction of myocardial infarct location, size and transmural extent at magnetic resonance imaging. Coron Artery Dis 18: 381-389.
- Kaandorp TA, Bax JJ, Lamb HJ, Viergever EP, Boersma E, et al. (2005) Which parameters on magnetic resonance imaging determine Q waves on the electrocardiogram? Am J Cardiol 95: 925-929.
- Rungroj K, Adisak M, Vithaya C, Pairash S, Olaree C, et al. (2009) Comparison of diagnostic and prognostic value of different electrocardiographic criteria to delayed-enhancement magnetic resonance imaging for healed myocardial infarction. Am J Cardiol 103: 464-470.
- Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, et al. (2002) American Heart Association Writing Group on Myocardial Segmentation and Registration for Cardiac Imaging. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. American Heart Association Writing Group on Myocardial Segmentation and Registration for Cardiac Imaging: A statement for healthcare professionals from the cardiac imaging committee of the council on clinical cardiology of the American heart association. Circulation 105: 539-542.
- Roger VL (2007) Epidemiology of myocardial infarction. Med Clin North Am 91: 537-552.
- Rosamond W, Flegal K, Furie K, Go A, Greenlund K, et al. (2008) American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 117: e25-146.
- Nichols M, Townsend N, Scarborough P, Rayner M (2014) Cardiovascular disease in Europe 2014: Epidemiological update. Eur Heart J 35: 2929.
- Roes SD, Borleffs CJ, Vander Geest RJ, Westenberg JJ, Marsan NA, et al. (2009) Infarct tissue heterogeneity assessed with contrast- enhanced MRI predicts spontaneous ventricular arrhythmia in patients with ischemic cardiomyopathy and implantable cardioverter-defibrillator. Circ Cardiovasc Imaging 2: 183-190.
- Anthony G, Michael E, Alfred E, Mark EJ, Kerry L, et al. (2001) Hafley. Prediction of long-term outcomes by signal-averaged electrocardiography in patients with unsustained ventricular tachycardia, coronary artery disease, and left ventricular dysfunction. Circulation 104: 436-441.
- Block P, Debels M, Goosens C, Konvens K, Taeymans Y, et al. (1990) Detection of late potentials on the averaged QRS signal recorded at rest and during a standardized stress test after acute myocardial infarction. J Electrocardiol 23: 144-149.
- Das MK, Khan B, Jacob S, Kumar A, Mahenthiran J (2006) Significance of a fragmented QRS complex versus a Q wave in patients with coronary artery disease. Circulation 113: 2495-2501.
- Das MK, Zipes DP (2009) Fragmented QRS: A predictor of mortality and sudden cardiac death. Heart Rhythm 6: S8-14.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences