A Case of Tumour Induced Osteomalacia Linked to a Hemangiopericytoma of the Palatine Fossa
Gebeily S, Gebeily SS, Khabouth J, Abbas MH, NassarA, Bechara C, Nehme J
DOI10.21767/2471-299X.1000017
1Neuroscience Research Center, Faculty of Medicine, Lebanese University, Beirut, Lebanon St. Joseph University faculty of Dentistry – Beirut, Lebanon
2Department of Oral Pathology, Faculty of Dentistry, St. Jospeh University, Beirut, Lebanon
3Department of Nephrology, The Lebanese Hospital, Geitawi – The Lebanese University Faculty of Medicine. Beirut, Lebanon
4Department of Neurology, The Lebanese Hospital, Geitawi – The Lebanese University Faculty of Medicine. Beirut, Lebanon
5Department of Radiology – The Lebanese Hospital, Geitawi – Beirut, Lebanon
6Department of Internal Medicine – The Lebanese Hospital, Geitawi, Beirut, Lebanon
7Department of Surgery – The Lebanese Hospital, Geitawi – Beirut, Lebanon
- *Corresponding Author:
- Gebeily S
Department of Neurology, The Lebanese Hospital, Geitawi, Beirut, Lebanon
Tel: +9611897767, +9613636265
Fax: +9611588581
E-mail: souheil.gebeily@gmail.com
Received date: Nov 25, 2015; Accepted date: Feb 19, 2016; Published date: Feb 24, 2016
Citation: Gebeily S,Gebeily SS, Khabouth J, Abbas MH, NassarA, Bechara C,A Case of Tumour Induced Osteomalacia Linked to a Hemangiopericytoma of the Palatine Fossa.Med Clin Rev. 2015, 2:8. doi: 10.21767/2471-299X.1000017
Copyright: © 2016 Gebeily S, Nehme Jet al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Tumor-induced osteomalacia (TIO) - also known as oncogenic osteomalacia - is a rare paraneoplastic syndrome characterized by phosphaturic osteomalacia linked to a phosphaturic mesenchyma tumour (PMT) which may induce renal phosphate wasting by excreting phosphatonin-like factors that will inhibit phosphate renal reuptake [1]. Most frequent clinical symptoms are muscular cramping, generalized weakness, chronic fatigue, bone pain, severe bone demineralization with increased risk of fractures. The concept of mesenchymal tumors associated with TIO was first considered as a possible single distinct entity was suggested in the early 1970’s by Evans, Azzopardi and Olefsky [2,3].
This clinicopathologic entity was further codified in 1987 by Weidner and Cruz as “Phosphaturic mesenchymal tumor, mixed connective tissue variant” (PMTMCT) who described 17 TIO-associated mesenchymal tumors, all showing similar histological features [4]. More recently, Folpe and colleagues established in 2004 that most of these tumours are in fact PMTMCTs [5]. Hemangiopericytomas are subtypes of PMTMCTs and the commonest TIO-associated tumors; they are usually benign slow-growing tumours; however, less frequently, malignant osteosarcoma and fibrosarcoma have been also reported [6].
Case Report
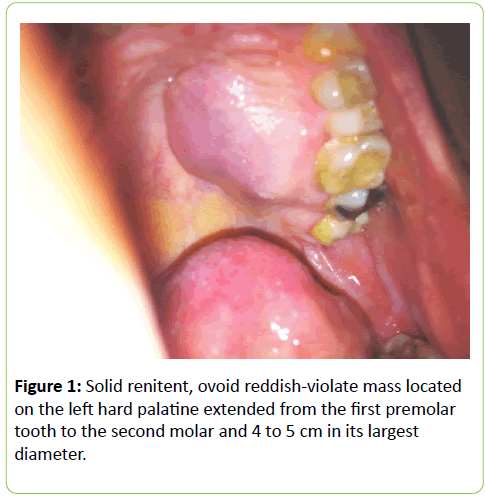
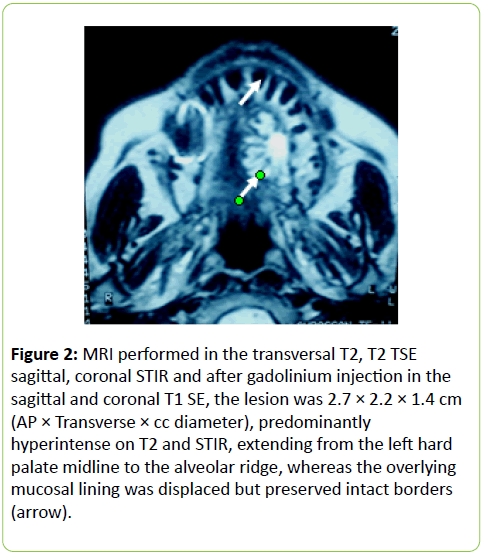
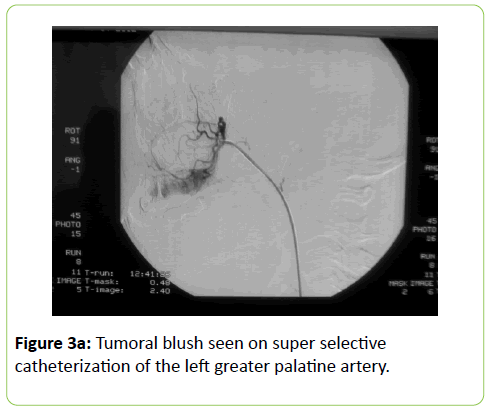
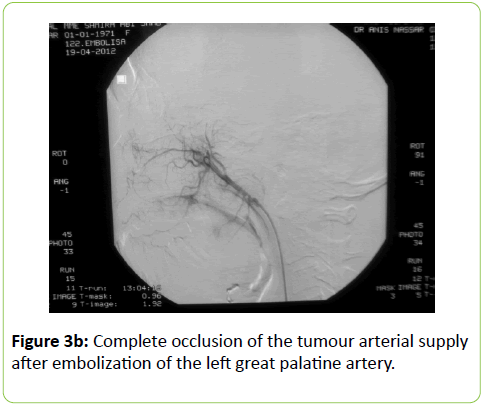
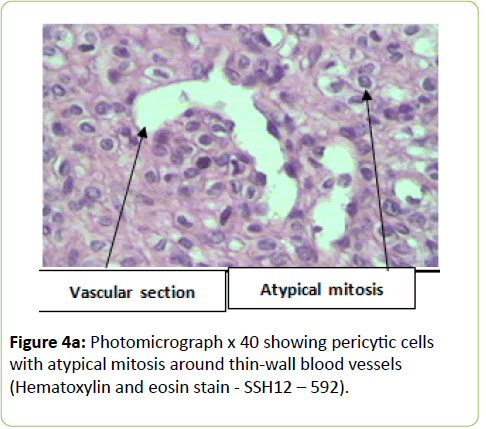
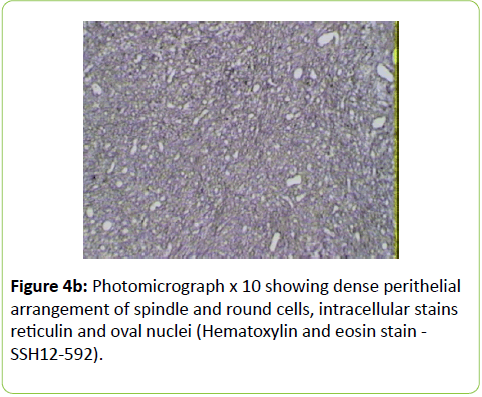
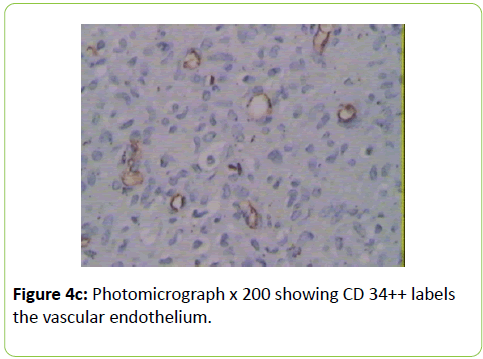
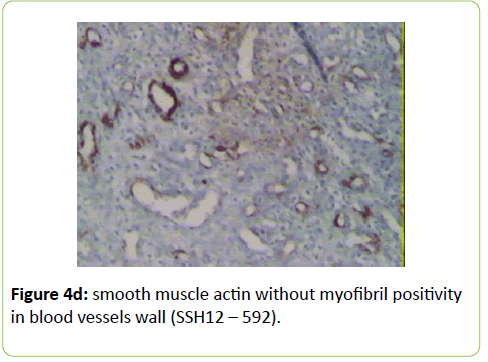
A 38 years old female patient started four years earlier to complain from persistent back pain, cramping, diffuse myalgia with progressive generalized weakness. A previous bone biopsy, showed an advanced stage of demineralization of non-specific nature that was reported to be linked to advanced stage of osteoporosis and though, she was treated with biphosphonate derivative (alendronate), calcium oral intake and cholecalciferol (Vit D3). She denied any familial history or personal comorbidities. She was referred to our neurological department on April 2011 for chronic fatigue, progressive lower limbs motor deficit with walking impairment, swallowing difficulty and diffuses myalgia. She also complained from chronic fatigue and weight loss. Incidentally, she reported the presence of a silent slow growing mass at the upper left palatine fosse, noticed five years earlier. On physical examination she was dysphonic with nasal voice tone and hypotonic soft palate, muscle tone was reduced, tendon reflexes were absent or reduced in the four limbs, muscle strength was scored 3 out of 5 in her lower limbs bilaterally with marked walking impairment as she could walk for limited distance with assistance. There was a well-defined ovoid reddish-violate solid, renitent mass, located on the left hard palatine extended from the first premolar tooth to the second molar and 4 to 5 cm in its largest diameter (Figure 1). Laboratory findings revealed severe hypophosphatemia (0.8 mg/dL ) despite high dose oral phosphorus intake (1 g/day), low serum calcium level (0.78 mg/dL), normal serum levels for alcaline phosphatase (ALP: 126 IU/L) and parathyroid hormone (PTHi 65 pg/ml); phosphaturia was abnomally high (1200 mg / 24 h) with normal calcium 94.5 mg/ 24 h, potassium (39 mMol/ 24 h), sodium 39 mMol / 24 h) levels in the urine; routine immuno-inflammatory tests were negative (ESR: 11 mm 1st hour, negative antinuclear antibodies (ANA) with normal profile screening tests, negative Anti-Neutrophil Cytoplasmic Antibody (ANCA), normal serum proteins electrophoresis with unremarkable immunoelectrophoresis profile; renal function was normal (serum creatinine 0.44 mg/dL, creatinine clearance 150 ml/min, microalbuminuria 44 mg/L). Nerve conduction study was within normal results except for overactive muscle fibres activity at rest, normal muscle fibre unit morphology ruling out any underneath myopathy. Biphosphonate was stopped; substitutive IV perfusion phosphorus therapy (0.08-0.16 mmol/kg) over 2-6 hrs for three consecutive days) increased the phosphorus serum level to 1.2 mg/dL, followed by high dose phosphorus and calcium oral intake and calcitriol (the active form 1,25 dihydroxyvitamin D-3); myalgia was significantly reduced, however, weakness and fatigue persisted unchanged. On CT scan there was a large mass arising from the left half hard palate showing thick bony trabeculae pointing from the bone into the lesion in a sunburst pattern, whereas several foci of bony thickening and bone erosion were seen. On the MRI performed in the transversal T2, T2 TSE sagittal, coronal STIR and after gadolinium injection in the sagittal and coronal T1 SE, the lesion was 2.7 ×2.2 × 1.4 cm (AP × Transverse × cc diameter), predominantly hyperintense on T2 and STIR, extending from the left hard palate midline to the alveolar ridge, whereas the overlying mucosal lining was displaced but preserved intact borders (Figure 2). The Echo-Doppler duplex scan, confirmed the marked tumoral hypervascularity. Considering the high risk of bleeding during surgery, a superselective catheterisation of the greater palatine artery was performed showing on arteriography a tumoral blush; selective embolisation procedure was performed on the tumor using small gelfoam particles achieving successfully the complete occlusion of the tumour and arterial blood supply (Figures 3a and 3b). On the next day, a total tumour resection with hemimaxilectomy was successfully performed. The diagnosis of Hemangiopericytoma was confirmed by histopathology and immunofixation techniques (Figures 4a-4d). A dramatic improvement was observed few days after surgery as the patient recovered rapidly her muscle strength with no report of any pain or myalgia.
Figure 2: MRI performed in the transversal T2, T2 TSE sagittal, coronal STIR and after gadolinium injection in the sagittal and coronal T1 SE, the lesion was 2.7 × 2.2 × 1.4 cm (AP × Transverse × cc diameter), predominantly hyperintense on T2 and STIR, extending from the left hard palate midline to the alveolar ridge, whereas the overlying mucosal lining was displaced but preserved intact borders (arrow).
Concomitantly, phosphatemia and urine phosphate returned to normal levels within 48 hours post-surgery. Two weeks later, she could walk independently with no limitation and without assistance. Follow up was uneventful since then and yearly CT.scan controls were also unchanged.
Discussion
Symptoms of hypophosphatemia are nonspecific and with variable underlying aetiology, duration, and severity. Mild hypophosphatemia (2-2.5 mg/dL) is generally asymptomatic; symptoms alone rarely alert the physician to the possibility of hypophosphatemia. Weakness, bone pain, rhabdomyolysis, and altered mental status are the most common presenting features in symptomatic hypophosphatemic patients [7]. Severe hypophosphatemia may induce generalized weakness, chronic fatigue, diplopia, dysphagia whereas the full-blown hypophosphatemic syndrome when the plasma phosphate concentration falls to 1.0 mg/dL (0.32 mmol/L) or less, seizures, respiratory depression, confusion, delirium and congestive heart failure may occur [8].
Metabolic-induced osteomalacia is usually diagnosed several years after first clinical symptoms when increased phosphaturia and low serum 1, 25 dihydroxyvitamin D levels are identified; however, in TIO, the diagnosis of phosphaturic mesenchymal tumours is more challenging and critical for the outcome and and chance for recovery. Our patient reported dysphagia as phosphatemia fell as low as 0.8 mg/dl; further delay in initiating surgery could have induced a more severe clinical presentation and a life-threatening situation. Extensive workup was performed excluding any other possible aetiology such as primary hyperparathyroidism, paraproteinemias, type II renal tubular acidosis (Fanconi syndrome) in the absence of renal glycosuria, aminoaciduria, and hypouricemia. X-linked Hypophosphatemic (XLH), and Autosomal Dominant Hypophosphatemic (ADHR) and TIO are group of diseases which are characterized by hypophosphatemia, hyperphosphaturia, without significant changes in serum concentrations of calcium, PTH, and 1,25(OH)2D3 and defective bone mineralization. XHL and ADRH were not considered in our case knowing the patient’s age, the late onset of clinical symptoms and the absence of any family history. Different histological types of mesenchymal tumours can be associated with the paraneoplastic TIO; these tumours are usually benign slow-growing vascular tumours; they are associated with a variety of histologic types and are commonly mesenchymal, of both smooth muscle and endothelial cells origin. The diagnosis of hemangiopericytoma is made according to distinct histological patterns which have been sub-divided into 4 types: 1) phosphaturic mesenchymal tumour, mixed connective tissue type (PMTMCT); 2) osteoblastoma-like tumours; 3) ossifying fibroma-like tumours; and 4) non ossifying fibroma-like tumors [4-6]. Hemangiopericytoma is the subtype of PMTMCT that comprises approximately 70% to 80% of all tumours associated with TIO and is usually an asymptomatic slow grow, benign tumour, explaining the delay in the diagnosis making; the diagnosis lasts a mean of 4.7 years from the onset of osteomalacia [5,9] From TIO reported cases in the literature, these tumours develop more frequently in the legs, shoulder, maxillo-facial, palate fossa, mandible, sinuses and nasal areas; rare cases are reported in the brain. Less frequently, malignant osteosarcoma and fibrosarcoma can be observed [10-20]. Phosphatonins are defined as circulating phosphaturic factors, among which the fibroblast growth factor (FGF23) is a bonederived hormone that regulates systemic phosphate homeostasis, vitamin D metabolism and inhibits renal tubular reabsorption of phosphate through mechanisms independent of PTH. Furthermore, FGF 23 interacts with the vascular endothelial growth factor (VEGF) inducing-regardless to its metabolic role - a mesodermic proliferation action by which it may act on the angiogenesis system as seen in hemangiopericytomas [21,22]. This phosphaturic - osteomalacia paraneoplastic syndrome is linked to a PMT which may excrete a phosphatonin-like factor that will inhibit phosphate renal reuptake and may induce renal phosphate wasting. Although FGF23 was not tested in our patient, the rapid normalisation of phosphatemia and phosphaturia combined with the dramatic recovery few days after surgical removal of the hemangiopericytoma highlights the probable implication of FGF23. The histological specific features showed dense perithelial arrangement of spindle and round cells, intracellular stains reticulin and oval nuclei; furthermore, CD34++ labels the vascular endothelium and smooth muscle actin (Figures 4a-4d).
Conclusion
The association of osteomalacia and symptomatic hypophosphatemia in the absence of renal insufficiency should path the way in identifying tumour induced osteomalacia and its underlying cause. Further research in the pathophysiology of TIO would highlight the relationship with FGF23 among other phosphatonin-like factors, particularly when hemangiopaericytomas are involved.
References
- Kumar K, Raghuveer KH (2008) Tumor-Induced Osteomalacia (TIO) (also Known as Oncogenic Osteomalacia). Radiol37:9-24.
- Evans DJ, Azzopardi JG (1972) Distinctive tumours of bone and soft tissue causing acquired vitamin-D-resistant osteomalacia. Lancet 1: 353-354.
- Olefsky J, Kempson R, Jones H, Reaven G (1972) "Tertiary" hyperparathyroidism and apparent "cure" of vitamin-D-resistant rickets after removal of an ossifying mesenchymal tumor of the pharynx. N Engl J Med 286: 740-745.
- Weidner N, Cruz SD (1987) Phosphaturic mesenchymal tumors. A polymorphous group causing osteomalacia or rickets. Cancer 59: 1442-1454.
- Folpe AL, Fanburg-Smith JC, Billings SD, Bisceglia M, Bertoni F, et al. (2004) Most osteomalacia-associated mesenchymal tumors are a single histopathologic entity: an analysis of 32 cases and a comprehensive review of the literature. Am J Surgpathol 28:1-30.
- Zadik Y, Nitzan DW (2012) Tumor induced osteomalacia: a forgotten paraneoplastic syndrome? Oral Oncol 48: e9-10.
- Schubert L, DeLuca HF (2010) Hypophosphatemia is responsible for skeletal muscle weakness of vitamin D deficiency. Arch Biochem Biophys 500: 157-161.
- O'Connor LR, Wheeler WS, Bethune JE (1977) Effect of hypophosphatemia on myocardial performance in man. N Engl J Med 297: 901-903.
- Dhammi IK, Jain AK, Singh AP, Mishra P, Jain S (2010) Oncogenic osteomalacia: Problems in diagnosis and long-term management. Indian J Orthop 44: 453-457.
- Gonzalez-Compta X, Mañós-Pujol M, Foglia-Fernandez M, Peral E, Condom E, et al. (1998) Oncogenic osteomalacia: case report and review of head and neck associated tumours. J LaryngolOtol 112: 389-392.
- Khadgawat R, Singh Y,Kansara S,Tandon N,Bal C,et al. (2009) PET/CT localisation of a scapular hemangiopericytoma with tumor-induced osteomalacia. Singapore Med J 50:255.
- Kawai Y, Morimoto S, Sakaguchi K, Yoshino H, Yotsui T, et al. (2001) Oncogenic osteomalacia secondary to nasal tumor with decreased urinary excretion of cAMP. J Bone Miner Metab 19: 61-64.
- Koriyama N, Nishimoto K, Kodama T, Nakazaki M, Kurono Y, et al. (2006) Oncogenic osteomalacia in a case with a maxillary sinus mesenchymal tumor. Am J Med Sci 332: 142-147.
- Viscasillas G,Maiz J,lao X,Zschaeck C,Sanz JJ (2005) Oncogenic osteomalacia cured by removal of an organized hematoma. EndocrPract 11:190-193.
- Kurien R, Manipadam MT, Rupa V (2010) Oncogenic osteomalacia in a patient with an ethmoid sinus tumour. J LaryngolOtol 124: 799-803.
- Pirola E, Vergani F, Casiraghi P, Leone EB, Guerra P, et al. (2009) Oncogenic osteomalacia caused by a phosphaturic mesenchymal tumor of the thoracic spine. J Neurosurg Spine 10: 329-333.
- Kenealy H,Holdaway I, Grey A (2008) Striking pathology gold: a singular experience with daily reverberations: sinonasal hemangiopericytoma (glomangiopericytoma) and oncogenic osteomalacia. Eur J InternMed 19:516-519.
- Brandwein-Gensler M,Siegal GP (2012) Striking pathology gold: a singular experience with daily reverberations: sinonasal hemangiopericytoma (glomangiopericytoma) and oncogenic osteomalacia. Head Neck Pathol6:64-74.
- David K, Revesz T, Kratimenos G, Krausz T, Crockard HA (1996) Oncogenic osteomalacia associated with a meningeal phosphaturic mesenchymal tumor. Case report. J Neurosurg 84: 288-292.
- Michi Y, Suzuki M, Kurohara K, Harada K (2013) A case of hemangiopericytoma of the soft palate with articulate disorder and dysphagia. Int J Oral Sci 5: 111-114.
- Fukumoto S (2008) Physiological regulation and disorders of phosphate metabolism--pivotal role of fibroblast growth factor 23. Intern Med 47: 337-343.
- Nelson AE, Bligh RC, Mirams M, Gill A, Au A, et al. (2003) Clinical case seminar: Fibroblast growth factor 23: a new clinical marker for oncogenic osteomalacia. J Clin Endocrinol Metab 88: 4088-4094.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences