Lp-PLA2, a Novel Potential Biomarker Predicting Cardiovascular Disease in Type 2 Diabetes Mellitus
Huolan Amelia Zhu
DOI10.21767/2471-299X.1000020
Cardiovacular Department, Shaanxi Provincials People’s Hospital, China
- *Corresponding Author:
- Huolan Amelia Zhu
Cardiovacular Department
Shaanxi Provincial People’s Hospital, China
Tel: 86-18992878761
E-mail: 294216621@qq.com
Received date: January 22, 2016; Accepted date: April 25, 2016; Published date: April 29, 2016
Citation: Zhu HA,Lp-PLA2, a Novel Potential Biomarker Predicting Cardiovascular Disease in Type 2 Diabetes Mellitus.Med Clin Rev. 2015, 2:11. doi: 10.21767/2471-299X.1000020
Copyright: © 2016 Zhu HA. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Cardiovascular disease (CVD) is the fatal complication of diabetes mellitus and it is a large economic burden of the world. Although there are a few biomarkers in predicting the cardiovascular disease, the disease is still far from being diagnosed and evaluated precisely in type 2 diabetes mellitus (T2DM) patients. Lp-PLA2 takes part in several links of the pathological mechanism in the diabetic cardiovascular disease. Researches have implied that the Lp-PLA2 has positive association with CVD severity in T2DM. Lp-PLA2 may be a novel biomarker diagnosing and evaluating CVD in T2DM.
Introduction
Nowadays, diabetes mellitus is sweeping the world as an epidemic. It has been a large burden of society [1]. Cardiovascular complication is the main cause of morbidity and mortality in diabetes patients. It is reported by World Health Organization, 50% of diabetes mellitus patients die from heart related disease, such as coronary heart disease, heart failure, ischemic heart disease, arrhythmia and sudden death [2]. Diabetes patients predispose to have cardiovascular disease, and more importantly, bear prone-rupture plague, leading to myocardial infarction and sudden death [3].
Biomarkers such as LDL-C, HDL-C have been well proved to predict CVD in population including T2DM patients. Some other traditional biomarkers have been applied in diagnosing and prognosing CVD, such as hs-CRP, IL-6, MCP-1, TNFα, VEGF [4]. There are also novel biomarkers especially reactive oxygen species raised up by the scientists, such as protein kinase C, nuclear factor-kB. Yet, there is still residual CVD hazard
remaining uncovered. Recent studies show that part of men and women with low 1-year predicted risk have subclinical CVD. The phenomenon is not paid enough attention to [5,6].
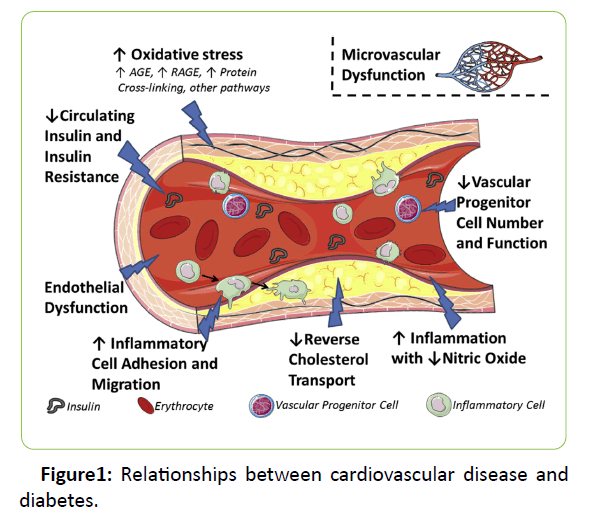
In order to predict the risk and prevent the development of cardiovascular disease in diabetes mellitus patients, it is essential to study the pathological mechanism of vasculature in diabetes mellitus. Several of the major mechanisms have been discussed for diabetic cardiovascular disease, including oxidative stress, progenitor cell dysfunction, microvascular dysfunction, and impaired reverse cholesterol transport (Figure 1) [7]. Among those process, Lp-PLA2, plays an important part in microvascular dysfunction, impaired reversecholesterol transport, and also oxidative stress.
Lp-PLA2 bio-chemical characteristic and physiology
Lp-PLA2, known as platelet activating factor acetylhydrolase (PAF-AH), belongs to Phospholipase A2 superfamily. Phospholipase A2 superfamily has five families: secretory PLA2 (sPLA2), cytosolic PLA2 (cPLA2), lysosomal PLA2; GVPLA2 (iPLA2) and lipoprotein-associated phospholipase A2. SPLA2 has several groups: IB, IIA, IIC, IID, IIE, IIF, III, V, X, and XI [8]. The Lp-PLA2 enzyme composes of 45 KDa molecular, secreted by monocyte, T lymphocyte, macrophages, mast cells, live cells, mostly by multiple inflammatory cells. Lp-LPA2 hydrolyzes fatty acids at sn-2 position into two polyunsaturated fatty acid and lysophospholipids which as marker of Lp-PLA2 activity, and specifically catalyzes the hydrolysis of oxidized LDL (ox-LDL) into lysophosphatidylcholine and oxidized fatty acid [9]. In the circulation, about 70% Lp-PLA2 binds to apoB transported by LDL and LP(a), apparently to the small dense LDL [10]. The other 30% binds to apoA1 transported by HDL. However, in diabetes mellitus patient, it is reported the distribution is altered [11]. Recent study reports that Lp-PLA2 regulates the pro-inflammatory and anti-inflammatory process [12].
Lp-PLA2 in the atherosclerosis process
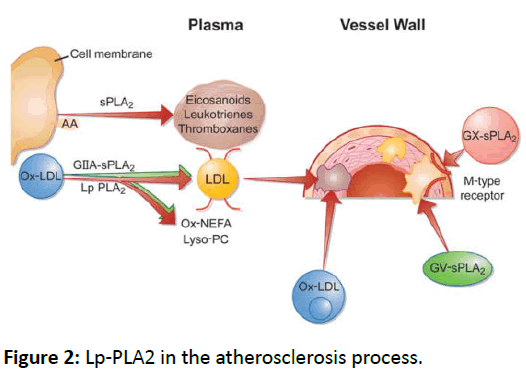
Due to the property of Lp-PLA2, it accumulates lysophosphatidylcholine in situ and induces the expression of several inflammatory cytokines [13]. MacRitchie [14] reports that Lp-PLA2 inactivates potent lipid messengers, such as PAF and modified phospholipids in settings of oxidant stress, and has the susceptibility to oxidative attack. In hypercholesterolemic pigs with high fat diet, De Keyzer points out that increased levels of oxidized LDL coming along with obvious increase in Lp-PLA2 activity [15]. In a 783 patients cohort study, it is reported Lp-PLA2 modulates LDL oxidation by hydrolysing oxidised phospholipids on particle surfaces. It is thought that the enzymes activity varies with genome type. It is strongly associated with genetic variants involved in lipid metabolism [16]. Under some circumstance, genetic depletion of Lp-PLA2 in mildly modified lipoproteins could enhance monocyte adhesion to endothelial cells comparing with lipoproteins that expressed normal activity levels [17]. In vitro experiment, exogenous Lp-PLA2 is able to inhibit endothelial cell apoptosis those induced by modified LDL particles (Figure 2) [18].
During the oxidative stress, lipoprotein-associated phospholipase A2 increases ox-LDL particles; and allows for enhanced internalization into the macrophage [19].
Studies on Lp-PLA2 in CVD and T2MD
A lot of epidemic studies have demonstrated that Lp-PLA2 is proposed as a predictive biomarker in CVD [20]. Mockel finds that Lp-PLA2 turns out to be a more effective risk marker than high sensitive CRP in acute coronary syndrome patients [21]. Also, by studying low risk coronary patients (classified by The Framingham risk score), Hargens discovers that Lp-PLA2 is significant independent predictor of those patients, while
traditional risk markers such as lipids, glucose are not. It is suggested that the enzyme may valuable in earlier detection of A lot of epidemic studies have demonstrated that Lp-PLA2 is proposed as a predictive biomarker in CVD [20]. Mockel finds that Lp-PLA2 turns out to be a more effective risk marker than high sensitive CRP in acute coronary syndrome patients [21]. Also, by studying low risk coronary patients (classified by The
Framingham risk score), Hargens discovers that Lp-PLA2 is significant independent predictor of those patients, while traditional risk markers such as lipids, glucose are not. It is suggested that the enzyme may valuable in earlier detection of subclinical CVD [22]. In 2015, Lp-PLA2 is shown associated with CVD severity, having a positive correlation with the number of atherosclerosis plagues and diseased arteries [23,24].
As known, CVD develops faster and more serious in diabetes patients than non-diabetes [25]. Resent studies keep on looking for circulatory predictors of CVD in Diabetes. Wootton discovers that Lp-PLA2 activity is an independent predictor of CHD risk in a sample of subjects with diabetes mellitus [26]. Research on diabetes pathogenesis discloses that Lp-PLA2 associates with insulin resistance and predicts incidents in Diabetes among older adults [27]. Some clinical studies indicate Lp-PLA2 is significantly elevated in diabetic CVD before balloon angioplasty. The increase mass and activity of Lp-PLA2 may lead to the high risk of CVD during the early course [28,29].
Early in 2005, Zalewski concluded an obvious relation between Lp-PLA2 activity and Diabetes [30]. In some latest studies, Garg discovers there is positive correlation between LP-PLA2 and fasting glucose in diabetes mellitus patients while in the same group, positive correlation between oxLDL and plasma glucose. And the author suggests that reducing the LPPLA2 might help decrease risk of CVD by interventing oxLDL in such group [29]. Nelson suggests that Lp-PLA2 activity is much more positive in T2DM than non-diabetes. Also the association is strongly positive in insulin resistance and Lp-PLA2 activity, and Lp-PLA2 mass is not positive in T2DM [27]. Fortunato concludes that Lp-PLA2 mass level is significantly higher in T2DM than non-diabetics, and the level is nearly 5 times higher than the normal group [28].
Hatoum concludes that LP-PLA2 activity is strongly related with incident CVD in T2DM, through observing 740 men and 777 women with confirmed diabetes enrolled in the Health Professionals Follow-Up Study and Nurses’ Health Study. The phenomenon is obvious in the first 6 years. The author suggests that the LP-PLA2 activity is more positively related with the unstable lesions than the atherosclerosis alone [31]. Kanzuo points out, serum Lp-PLA2 activities has a positive relation with lysophosphatidylcholine contents in LDL in T2DM patients. Lysophosphatidylcholine is the production of Oxidized phospholipids in LDL hydrolyzed by Lp-PLA2.
Lysophosphatidylcholine is increased in T2DM patients than the nondiabetic ones [32].
However, there is a contrast data against that LP-PLA activity and mass has positive relation with CVD. Nelson claims that the activity and mass has no difference between diabetes and non-diabetes group than expected [33].
Discussion
It is essential for the medical industry to prevent the CVD in diabetes mellitus before struggling to find solution when it becomes severe. In order to diagnose the CVD in the early stage and evaluate the severity, circulating biomarkers are important parts in the procedure. Although there are a few biomarkers, it is still far from expectation. Lp-PLA2 is a novel biomarker empathized these days. A Lot of evidence proves Lp-PLA2 to be a strong marker predicting the severity and outcomes of cardiovascular disease.
The endothelial cell layer is a barrier separating circulating stuff from the arterial intima. It is reported that macrophage infiltration enhances in diabetic mellitus patients’ arterial wall [30]. Lp-PLA2 is secreted by macrophages mostly. Macrophage infiltrates the adipose tissue producing oxidative byproducts and also increases the production of Lp-PLA2. The free radical from oxidation of excess glucose could increase the oxidation of lipoproteins and therefor increase the substrate for Lp- PLA2. Whether Lp-PLA2 contributes to the oxidation itself or the just the byproduct, It means that Lp-PLA2 could imply the inflammation level. José L reports that intensified
hypoglycemic therapy could decrease total Lp-PLA2 activity and redistribute Lp-PLA2 activity toward a higher proportion in high-density lipoprotein. The phenomenon hints Lp-PLA2 activity enhance in hyperglycemic people who intend to have high risk of CVD [34].
The Lp-PLA2 may be a crucial biomarker in diagnosing and evaluating CVD in diabetes mellitus patients. The pathological mechanism of cardiovascular disease in diabetes performs quite similar as in normal population, but it also has its own peculiarity. The Lp-PLA2 should be a potent biomarker in evaluating diabetic CVD, because the enzyme contributes to oxidative stress, progenitor cell dysfunction, microvascular dysfunction, and impaired reverse cholesterol transport. Recently, clinical researches already show encouraging results. Also, diabetic metabolic patients have higher levels of small dense LDL [35], which potentially offers better matrix for Lp- PLA2 transporting in circulation. Researches have proven it.
In conclusion, it is still essential to seek novel biomarker in order to prognose and diagnose the CVD in T2DM. Lp-PLA2 takes part in several process of atherosclerosis in diabetes patients. A lot of researches have proven that Lp-PLA2 might be a promised biomarker.
References
- Chan JC, Zhang Y, Ning G (2014) Diabetes in China: a societal solution for a personal challenge. Lancet Diabetes Endocrinol 2: 969-979.
- (2012) New WHO statistics highlight increases in blood pressure and diabetes, other noncommunicable risk factors. Cent Eur J Public Health20: 134-149.
- Chiha M, Njeim M, Chedrawy EG (2012) Diabetes and coronary heart disease: a risk factor for the global epidemic. Int J Hypertens 2012: 697240.
- Shoamanesh A, Preis SR, Beiser AS, Vasan RS, Benjamin EJ, et al. (2015) Inflammatory biomarkers, cerebral microbleeds, and small vessel disease: Framingham Heart Study. Neurology 84: 825-832.
- Folsom AR (2013) Classical and novel biomarkers for cardiovascular risk prediction in the United States. J Epidemiol 23: 158-162.
- Michos ED, Nasir K, Braunstein JB, Rumberger JA, Budoff MJ, et al. (2006) Framingham risk equation underestimates subclinical atherosclerosis risk in asymptomatic women. Atherosclerosis 184: 201-206.
- Kovacic JC, Castellano JM, Farkouh ME, Fuster V (2014) The relationships between cardiovascular disease and diabetes: focus on pathogenesis. EndocrinolMetabClin North Am 43: 41-57.
- Burke JE, Dennis EA (2009) Phospholipase A2 biochemistry. Cardiovasc Drugs Ther 23: 49-59.
- MacPheeCH, Moores KE, Boyd HF, Dhanak D, Ife RJ, et al. (1999) Lipoprotein-associated phospholipase A2, platelet-activating factor acetylhydrolase, generates two bioactive products during the oxidation of low-density lipoprotein: use of a novel inhibitor. Biochem J338:479-487.
- Gazi I, Lourida ES, Filippatos T, Tsimihodimos V, Elisaf M, et al. (2005) Lipoprotein-associated phospholipase A2 activity is a marker of small, dense LDL particles in human plasma. ClinChem 51: 2264-2273.
- Kujiraoka T, Iwasaki T, Ishihara M, Ito M, Nagano M, et al. (2003) Altered distribution of plasma PAF-AH between HDLs and other lipoproteins in hyperlipidemia and diabetes mellitus. J Lipid Res 44: 2006-2014.
- Stafforini DM (2009) Biology of platelet-activating factor acetylhydrolase (PAF-AH, lipoprotein associated phospholipase A2). Cardiovasc Drugs Ther23: 73-83.
- Shi Y, Zhang P, Zhang L, Osman H, Mohler ER,et al. (2007) Role of lipoprotein-associated phospholipase A2 in leukocyte activation and inflammatory responses. Atherosclerosis 191: 54-62.
- MacRitchie AN, Gardner AA, Prescott SM, Stafforini DM (2007) Molecular basis for susceptibility of plasma platelet-activating factor acetylhydrolase to oxidative inactivation. FASEB J 21: 1164-1176.
- De Keyzer D, Karabina SA, Wei W, Geeraert B, Stengel D, et al. (2009) Increased PAFAH and oxidized lipids are associated with inflammation and atherosclerosis in hypercholesterolemic pigs. ArteriosclerThrombVascBiol 29: 2041-2046.
- Suchindran S, Rivedal D, Guyton JR, Milledge T, Gao X, et al. (2010) Genome-wide association study of Lp-PLA(2) activity and mass in the Framingham Heart Study. PLoS Genet 6: e1000928.
- Lee C, Sigari F, Segrado T, Horkko S, Hama S, et al. (1999) All ApoB-containing lipoproteins induce monocyte chemotaxis and adhesion when minimally modified. Modulation of lipoprotein bioactivity by platelet-activating factor acetylhydrolase. ArteriosclerThrombVascBiol19:1437-1446.
- ChenCH, Jiang T, Yang JH, Jiang W, Lu J, et al. (2003) Low-density lipoprotein in hypercholesterolemic human plasma induces vascular endothelial cell apoptosis by inhibiting fibroblast growth factor 2 transcription. Circulation107:2102-2108.
- Rosenson RS, Hurt-Camejo E (2012) Phospholipase A2 enzymes and the risk of atherosclerosis. Eur Heart J 33: 2899-2909.
- Rosenson RS (2010) Phospholipase A2 inhibition and atherosclerotic vascular disease: prospects for targeting secretory and lipoprotein-associated phospholipase A2 enzymes. CurrOpinLipidol21:473-480.
- Mockel M, Muller R, Vollert J, Muller C, Danne O, et al. (2007) Lipoprotein-associated phospholipase A2 for early risk stratification in patients with suspected acute coronary syndrome: a multi-marker approach: the North Wuerttemberg and Berlin Infarction Study-II (NOBIS-II). Clin Res Cardiol96: 604-612.
- Hargens TA, Rhodes PG, VanReenen J, Kaminsky LA (2014) Lipoprotein-associated phospholipase A2 and carotid intima-media thickness in individuals classified as low-risk according to Framingham. CardiovascDiagnTher4: 487-494.
- Celik O, Ozturk D, Akin F, Satilmis S, Yalcin AA, et al. (2015) Evaluation of lipoprotein-associated phosholipase A2 and plaque burden/composition in young adults. Coron Artery Dis 26: 266-271.
- Cai A, Li G, Chen J, Li X, Li L, et al. (2015) Increased serum level of Lp-PLA2 is independently associated with the severity of coronary artery diseases: a cross-sectional study of Chinese population. BMC CardiovascDisord 15: 14.
- Naito R, Kasai T (2015) Coronary artery disease in type 2 diabetes mellitus: Recent treatment strategies and future perspectives. World J Cardiol 7: 119-124.
- Wootton PT, Stephens JW, Hurel SJ, Durand H, Cooper J, et al. (2006) Lp-PLA2 activity and PLA2G7 A379V genotype in patients with diabetes mellitus. Atherosclerosis 189: 149-156.
- Nelson TL, Biggs ML, Kizer JR, Cushman M, Hokanson JE, et al. (2012) Lipoprotein-associated phospholipase A2 (Lp-PLA2) and future risk of type 2 diabetes: results from the Cardiovascular Health Study. J ClinEndocrinolMetab 97: 1695-1701.
- Fortunato J, Blaha V, Bis J, Stasek J, Andrys C, et al. (2014) Lipoprotein-associated phospholipase Aâ‚‚ mass level is increased in elderly subjects with type 2 diabetes mellitus. J Diabetes Res 2014 .
- Garg S, Madhu SV, Suneja S (2015) Lipoprotein associated phospholipase A2 activity & its correlation with oxidized LDL &glycaemic status in early stages of type-2 diabetes mellitus. Indian J Med Res141: 107-114.
- ZalewskiA,Macphee C (2005) Role of lipoprotein-associated phospholipase A2 in atherosclerosis: biology, epidemiology, and possible therapeutic target. ArteriosclerThrombVascBiol25: 923-931.
- Hatoum IJ, Hu FB, Nelson JJ, Rimm EB (2010) Lipoprotein-associated phospholipase A2 activity and incident coronary heart disease among men and women with type 2 diabetes. Diabetes 59: 1239-1243.
- Sonoki K, Iwase M, Sasaki N, Ohdo S, Higuchi S, et al. (2009) Relations of lysophosphatidylcholine in low-density lipoprotein with serum lipoprotein-associated phospholipase A2, paraoxonase and homocysteinethiolactonase activities in patients with type 2 diabetes mellitus. Diabetes Res ClinPract86: 117-123.
- Nelson TL, Kamineni A, Psaty B, Cushman M, Jenny NS, et al. (2011) Lipoprotein-associated phospholipase A(2) and future risk of subclinical disease and cardiovascular events in individuals with type 2 diabetes: the Cardiovascular Health Study. Diabetologia 54: 329-333.
- Sanchez-Quesada JL, Vinagre I, de Juan-Franco E, Sánchez-Hernández J, Blanco-Vaca F, et al. (2012) Effect of improving glycemic control in patients with type 2 diabetes mellitus on low-density lipoprotein size, electronegative low-density lipoprotein and lipoprotein-associated phospholipase A2 distribution. Am J Cardiol110: 67-71.
- Eckel RH, Wassef M, Chait A, Sobel B, Barrett E, et al. (2002) Prevention Conference VI: Diabetes and Cardiovascular Disease: Writing Group II: pathogenesis of atherosclerosis in diabetes. Circulation 105: e138-e143.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences