An Overview of Fluoride Toxicological Profile, Pollution Aspects and Remedial Solutions
Rajendra S Dongre
Rajendra S Dongre*
Department of Chemistry, R.T.M. Nagpur University, Nagpur, India
*Corresponding Author:
Rajendra S Dongre
Department of Chemistry, Nagpur University, Nagpur, India
E-mail: rsdongre@hotmail.com
Received Date: 08-25-2020; Accepted Date: 09-21-2020; Published Date: 09-28-2020
Citation: Rajendra S Dongre (2020) An Overview of Fluoride Toxicological Profile, Pollution Aspects and Remedial Solutions. Med Clin Rev. Vol. 6 No. 4: 102.
DOI: 10.36648/2471-2120X.6.4.102
Abstract
Undue fluoride is recited in all atmospheric domains including water throughout the globe especially Indian continent where utmost region endangered endemic fluorosis. Indeed fluoride contaminations in air, water and land are derived through assorted causes viz; granite rocks dissolution, percolations during rains and manmade activities. Such high fluoride pollution poses acute toxicity in nature along with detrimental effects on health like bone/dental deformations, skeletal and Non skeletal fluorosis, even damage to male reproductive system. Rampant fluorosis raised due to excessive fluoride contaminations gets mitigated through viably developed de-fluoridation/fluoride removal water purification techniques. Subsequently assorted technologies are developed for water fluoride removal/defluoridation viz; adsorption, precipitation, coagulation, ion-exchange, electrolyticdialation/ electrodialysis and membrane-separations, yet crisis are unresolved. This research is an overview of fluoride and its toxicological profile and pollution aspects besides remedial solutions like fluoride exclusion by means of holy basil/ tulasi leaves, crush limestone and other prominent fluoride adsorbents also its advantages and disadvantages are discussed. State-of-the-art fluoride removal technologies are summarized that aids to reduce excess fluoride contaminations in the water.
Keywords
Fluoride; water; Fluorosis; De-Fluoridation; Chitosan; Bio-Composites; Adsorption
Introduction
“Without man, water subsists; however life exists for a while without water”.
“Abundant water is cheap, yet safe pure water is scare & fewer for mere good health”
Above slogan cites significance of water in life and its vitality for sustainable nourishment with copious favours [1]. Nature’s subsistence depends on water as and life on planet can’t exist besides difficult to survive without water [2]. Amid, water quality is still a great concern throughout the world as resources are getting contaminated by both natural and manmade activities ultimately affects health as well as socio-economic growth. In 1980, UNICEF identified, addressed and launched technology mission programme for surplus fluoride contamination including endemic fluorosis to provide fluoride-free and safe drinking water besides strengthened/facilitated monitoring systems for sustaining water quality [3].
Among known utmost environmental pollutants, arsenic and fluoride alone have deteriorated water quality that does threaten health of billions thus seek attempts to remediate fluoride contamination [4]. Health hazardous due to excess water fluoride gets global problem in Africa, Europe and Asian countries including India. The Figure 1 below depicts the epidemic fluorosis affected regions in India [4-6]. Further, the fluoride contaminated groundwater in Indian state is shown in Figure 2 below.
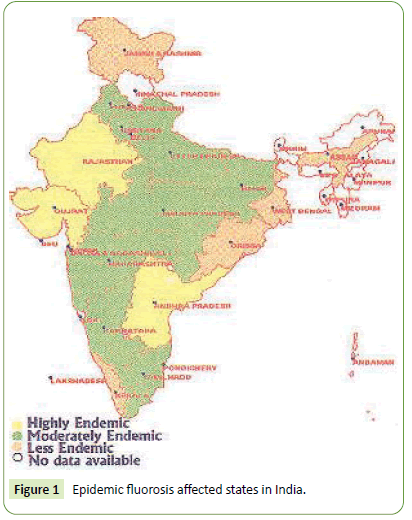
Figure 1: Epidemic fluorosis affected states in India.
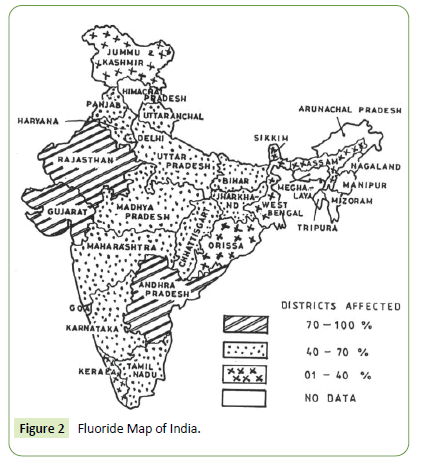
Figure 2: Fluoride Map of India.
This excess fluoride contamination in groundwater and other water sources becomes serious as billion populations are found affected in India and worldwide too. WHO national standard (ES 261:2001) permits 1.5 ppm safe fluoride limit for consumption, yet drinking water exceeds fluoride than its permissible level [7]. Various pollutants including heavy metals; organic compounds along with its stringent limit, its potential sources and corresponding health effects of possible contaminant in water as per EPA water regulations are depicted in Table 1 below [4-9].
Table 1. Potential sources and corresponding health effects owing stringent limit of various pollutants vide EPA water regulations.
All such chemical contaminations found to create crucial risk and instant serious impacts on human health and ecological systems. Fluoride concentrations <0.3 mg/g is found in surface water whilst groundwater holds much higher fluoride contaminations of 10 mg/g by virtue of rock-sediment interactions. Earth’s crust contains fluoride salts contents about 300 mg/kg as granite gneisses, pegmatite, fluorite ore, gypsum and fluorite base coal-evaporates further defiles water contaminations. Rather, certain inorganic and organic derived dissolved salts established excessive fluoride contamination, boosted water salinity of the order of <0.05 %.
Toxicological Functions of Fluoride
Physico-chemical outline
Fluorine with atomic weight 18.9 is the 1st element of halogen group VII-A, was discovered in 1886. The Latin nous of fluorine is "to flow". Fluorine is highest electronegative, reactivity highly poisonous, pale yellow prickly odour gas rarely occurred elemental in nature due to instant reactivity. Fluorine rather as fluoride/F- amount as 0.3 g/kg in earth's crust and counted as the 13th abundance. Fluoride reacts faster with certain heavy metals like iron, aluminium and manganese so as to yield assorted naturally spread compounds. Fluoride is organoleptic neutral hence needs to test and monitored in systems [7].
Fluoride usage in Industries
The nuclear ventures and certain atomic bomb explosions utilizes many fluorine compounds in routine R&D. Hydrogen fluoride is industrially used dehydrating agents and used for itching of glasses get source for atmospheric contaminations. Assorted organic fluorides are used as crude/raw materials for various purposes viz; biomedical, agriculture, pesticides, and insecticide usages. Strong and inert C-F resistive chemical degradation bond are used commonly as CFC agents in refrigerant, plastic, pharmaceutics, oil, pesticide and insecticides. Fluoride salts of calcium, magnesium and sodium are used in toothpastes and mouthwash gets poisonous above its stringent amount. Fluoride additives can protect enamels, dental nourishments and boosts teeth stability/strengthen via evading native cavities. Assorted medicines, drugs and vital API are developed for assorted health and diseases treatments found to incorporate fluorides in its synthetic routes also contributes excess contaminations. Organo/ aromatic fluoro derivatives acted imperative drug components in variety of ailment treatments and therapies. Prozac drugs utilize fluorine derivatives which found to stay prolonged in body prior its metabolic breakdown via fat dissolution. This fluoride shows drug selectivity and facilitates dissolution of lipids/fats which reduces its metabolism. About 35% antibiotic, anti-inflammatory, anti-depressant, cholesterol- lowering and anaesthetic drugs can use fluoride in API making. Consequently assorted fluorine based biomolecules are developed for innate beneficial medicinal/ clinical usages. Fluoride based pharmaceutics, diagnostics and agrochemicals have involved fluorine derived natural-scaffolds owing site-selective bio-activities. These fluorinated templates own extensive significance due to unique features viz; utmost electro-negativity, alter electron distribution which influences bio-absorption, distribution, overall metabolism and in-vitro bio-activities for diverse enzymes. Excess fluoride contamination in water/diet tempt Na+, K+ directed enzyme inhibitions like adenosine triphosphatase (Na+,K+-ATPase) deactivation beside many patho-physiology disorders and viable neural health problems [4-6] are shown in Figure 3 below.
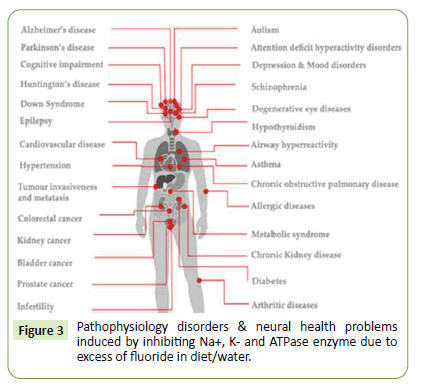
Figure 3: Pathophysiology disorders & neural health problems induced by inhibiting Na+, K- and ATPase enzyme due to excess of fluoride in diet/water.
Fluoride’s ecological output
Fluoride and its derivatives embedded ecological damages by virtue of inertness C-F linkage, ultimately persists in the environment for long durations. High fluoride found to accrue in food chains which appears toxic and sometimes lethal e.g., plants/vegetation and subsequently in insects, birds, and mammal eating plants. Fluoride pollution in water is also observed due to untreated industrial, sewage and domestic wastewaters being magnified as stirred up in food-chains. Excessive fluoride contaminations have induced in atmosphere by means of assorted industrial activities like aluminium/metal smelting, phosphate processing, electronics and steel/brick/tile/clay/glass makings besides coal combustions. Certain organo-fluorine based agrochemicals and intermediate products have applied in extreme amount for crop growth and agricultural maintenance can generates viable fluoride pollution via percolated rain water in ecological bodies. Industrial sources including high-tech semiconductor built-up, electronics and integrated circuits found to acquit excessive atmospheric fluoride pollution of 16000 tons per annum [6].
Fluoride induce water pollution
Most water bodies found contaminated by excess watersoluble fluorides which gets originate via mineral, ore, rock and coal activities. Factory scrubbers and sewage treatment effluents contain dissolved fluorides end up in water bodies like streams, lakes, ponds, wells and rivers. Such dissolve fluoride contaminations distressed several aquatic/ocean organisms/ species and flora-fauna. Thus most waters are unsafe for consumptions due to geogenic originated fluoride contamination through ore, mineral and rock leaches beside soil, water and air contaminations further deteriorated water quality. Certain geochemical factors like volcanic eruptions and plutonic activities have further boosted fluoride intrusion in water bodies and food-chain systems [4-9]. The earth’s upper crust contains raw fluorides which viably dissolute water, causes surface and groundwater pollutions. The groundwater fluoride contamination are segregated by geological, chemical and physical parameters of aquifer which further depend on assorted factors like porosity, acidity, rocks quality, temperature, and soil depth etc. The wellwaters in many states of India have reported 35 ppm of fluoride amount quite higher than international standards Table 2 [7].
Table 2. Fluoride stringent level in water as per assorted international standards.
Fluoride exposure: Sources & Scale
Environment: Fluoride concentration in notable ores and minerals are given in Table 3 [4]. The principal minerals called fluorspar, sellaite, cryolite, villianmite and fluorapatite are basically water insoluble as exist in sedimentary and igneous rocks can pollute ground waters in favourable dissolution status. About 95% fluoride deposits in ore/mineral in the form of ionic salts of Ca, P, Ce, La, Mg and Na alkali and alkaline metals. Ground water is more facile to leaching excessive fluoride contamination through rocks than that of surface waters. Rock phosphates enter via huge mining activity and elemental raw feedstock for phosphorus derived fertilizers viably contributes excess fluoride contaminations in water.
Table 3. Fluoride concentration in minerals & ores.
Artificial Sources: Besides anthropogenic factors, fluoride also gets released and contributed water contamination via artificial sources and activities including raw coal combustion, industrial wastewaters and ore/mineral processing [4]. Hydrogen fluoride production through Na3AlF6, AlF3 usages, gasoline alkylates and chlorofluorocarbons, etching semiconductors, glasses, brick cleaning, tanning leather and rust remover progress carries undue fluoride contaminations in its process effluents. CF2 is common feedstock/ raw material and flux agent in many processes like steel, glass and enamel, HF and electrolytic production too imparts fluoride contaminations. Fluoridating agent like H2SiF6, Na2SiF6 and NaF are used as a preservative in glues; glass, wood preservative besides SF6 usage in electronic components gives fluoride contamination. Such synthetic chemicals induce further fluoride pollution in our environments [6,7].
Other sources of Fluoride pollution: Many other significant contributions are observed for fluoride consumption/uptake by humans including cigarette consumptions, Teflon-coated cookware usages, stainless steel/pyrex ware also own excess fluoride level. Fluorides leached in soils are viable for fluorosis due to grazing of fluoride up-taken plants/vegetables by human and animals. Important vegetation/forage by assorted factors like fluoride-rich effluents, windblown and rain-splashed, a combination thereof gets susceptible contributions. Certain preserved foods, soft drinks, fruit juices, animal milk found to contain excess fluoride amount in its contents may also induced fluoro-pesticide residual water used during their manufacturing [6-9].
Fluoride: Pollution aspects
Fluoride & human contact: Habitation found exposed to high fluoride concentration via water, food, air, medication/drugs, fertilizers, pesticides, insecticides and cosmetic etc.
•Water: About 60% of assailable fluoride toxicity occurs through drinking water.
•Food: Food/crop uses assorted fertilizers, pesticides and insecticides which in incomplete consumptions appear in air, land and water ultimately food-chain in varied proportions. Crude/ raw feedstock/starting materials utilized in production/making of calcium carbonate, talc and chalk induces excess fluoride concentrations about 800-1000 mg/g in its contents.
•Drugs: The sodium fluoride usage in treatment of osteoporosis, Niflumic acid in treatment of rheumatoid arthritis, mouth-rinse/ wash delivered chronic adverse effects of fluoride.
•Air: Metal processing, fertilizers, agrochemicals industries gives facile fluoride exposure to their workers and nearby habitants during its manufacturing.
Fluoride’s ecological movement, circulation and alteration: Fluorides found in atmosphere as gaseous and particulate nature which can travel a long distances due to air/wind and atmospheric turmoils. Certain fluoride chemicals can persists in environment for hundreds of years right from troposphere spear and then migrates to stratosphere. Such ecological movements, circulations and alterations of fluorides in air, water and soil gets affected by pH, hardness and incident clays/minerals. Anionic fluoride anions undergo complexation with metallic cations in the surrounding atmosphere and endure extensive transport via water/air cycle which gets viable in resultant food-chains. Fluoride adsorption in land-phase is profoundly enhanced at moderate acidic pH and found deposited in soils for long span. Aquatic organisms and earthly biota uptakes fluoride alters extremely through exposures and its bioavailability besides excretion phenomenon. Widespread fluoride contamination is occurred through coal burns in domestic and thermal/power plant usages as common practices in many Asian countries including Pakistan, India and China. Industrial fluoride containing effluents found discarded into waterways or poorly treated gets easily reach into environment and originate serious contaminations. Such fluoride water/solid-wastes indecent disposal carries leakage/crop-up in soil/land and ultimately damages the habitants exposed at high levels/periods.
Fluoride effect on plants: Plants and vegetables can intake excess fluoride in its roots and leaves through the soil water, and air routes. Such overdose of fluoride brings alterations in metabolism as getting adverse to carry out leaf chlorosis/necrosis, shrink developments, less, production/yield and death in certain extremes. Certain species of plants are more sensitivity to fluoride and cannot tolerate excessive fluoride contaminations/ interferences. The plants act as a dietary source for animals and human hence it’s indispensible to control excessive fluoride intrusion in these species to keep healthy and sustainable food-cycle.
Fluoride effects on animals: Plants are dietary sources for animals and human so, excessive fluoride uptake by plant many leads augmented exposures to both man and animals which consumes it in their daily food-chains. There are certain practically-- induced persistent toxicity as induced by excessive fluoride amount which grows endemic skeletal, non-skeletal and dental fluorosis issues. Investigational chronic/acute toxicity analysis revealed certain problems owing non-specific symptoms found to impart nephrotoxicity in many rodents. Fluoride contamination/ intrusion in animals are not reporting direct mutagenicity in animals, instead adversely modifies mutagen based responses.
Fluoride detrimental effects on man: Since 1930s scientific documents have revealed clinical data indicated fluoride inhibitions to enzyme strain viable for acid-producing oral bacteria which consumes enamel and thus fights with dental cavity. Fluoride and calcium ionic binding at limiting scale further reinforce tooth enamel while extra fluoride ingestion losses calcium from tooth template and worst case causes dental fluorosis rather than remedying cavities. Recurring fluoride consumption origins crippled skeletal fluorosis due to persistent fluoride in tissues and fluids of humans. Diets owing controlled fluoride amount found certain beneficial effects like endorsement of growth in rodents, boosts fertility, and lessens anaemia. Fluoride also carries nucleation, precipitation and crystallization of bone apatite hence at guarded limit is vital for our life while higher level imparts cariostatic effect. Fluoridecalcified accumulation found in soft tissues which affects renal excretion induced glomerular filtration and tubular re-absorption altering urinary flows [7-10]. Recommended dietary allowances are estimated safe and sufficient for fluoride intake by human at various stages of life is given in following Table 4.
Table 4. Recommended daily dietary intakes of fluoride.
Fluoride carcinogenicity: Fluoride acts as a carcinogen agent that can advances carcinogenesis and induced cancer due to damage of genomes and disordering cellular metabolism. Cancer rates are reported in many species those getting exposed to recurring and excessive fluoride amounts. Fluorspar mining and aluminium manufacturing workers are susceptible for fluoride are mixed exposures which tempts many carcinogenesis phenomenon viz; lung/pulmonary, pancreatic, genitourinary and lympho haematopoietic malignancy. Carcinogenicity of prolonged fluoride disclosures are also reported for cancer mortality rates in many epidemiology analyses. The cryolite mineral processing industry owing excessive fluoride dust has revealed augmented bladder cancer cases in their workers [9].
Fluorides teratogenicity: Fluoride also acts as teratogen agent and upsets growth of embryo/foetus stage during pregnancy which also brings out birth defects like congenital malformation. Excessive fluoride consumptions carry out adverse foetal developments beside prematurity in many cases. Amplified Down's syndrome pattern is observed with high fluorides in drinking water in Massachusetts USA during 1950-66 besides in Sweden during 1968-77. Certain fluoride induced biological effects are given in Table 5 [7-14].
Table 5. Concentrations of fluorides and biological effects.
Fluorosis preventive and awareness: Community awareness and health education is to be given to combat endemic fluorosis. Adverse effects of fluoride pollutions are unavoidable yet alertness, health educations besides rationally developed fluoride removal/de-fluoridation treatment technologies as instant and safe remedy to reduce fluorosis problem/risk. The assorted intensive campaigns, nutrition, health instructions and motivate society contributions are some easier ways to combat with fluoride induced toxicity. The government must generate public consciousness and provides healthiness education for endemic fluoride affected area public/society and seek sole involvement through interceptive programmes. Media, TV and government agencies along with NGO should educate public/layman in local idiom and dialect for fluoride poisoning/fluorosis owing below mentions preventives [4]:
• Aware for role of fluoride in water/life and brief signs & symptoms of fluorosis.
• Identify viable sources of fluoride pollutions
• Certify and provide dietary advices
• Furnish preventive measures for de-fluoridation/fluoride removal techniques
• Categorize risk scale conditions in citizens and give medical treatments
The indicative fluorosis symptoms are summarized in flow chart as shown in Figure 4 below [4-7]:
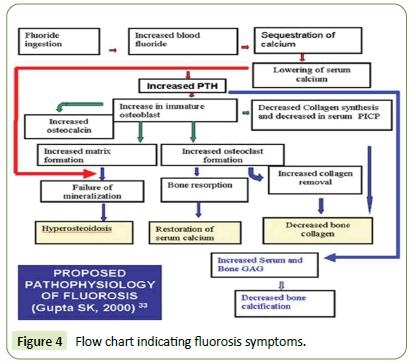
Figure 4: Flow chart indicating fluorosis symptoms.
Nutritional Intervention/Diets for Fluorisis mitigation:
a) Role of Diet & Vitamins for Fluoride alleviation: The clinical data signified assorted nutritional based effective remedies including adequate vitamin-C, metal ions like calcium and magnesium ingestion found to decreases fluorosis. Vitamin- C/D consumption of 50 ppm/day reduces fluoride-toxicity viz; deprived weights, hardening/softening of tissue; reduce bone and teeth density besides carbohydrate/fat metabolic disorders. Ascorbic acid rich dietary intake ameliorates side-effects of excessive fluoride consumptions, vita-C acts as co-enzyme for collagen/amino acids called proleins hydroxylation which is vital for consolidation of bone and tooth matrix. Thus vitamin-C/ ascorbic acid rich food-stuffs like amla, citrus fruits and vegetables as dietary and nutritional supplements along with vitamin D aids to combat skeletal fluorosis. This wrathful nutritional prophylaxis and co-administered vitamin-D and calcium based therapy initiated beneficial effects in fluoride induced of osteoporosis issues. Research performed in Rajasthan state of India have advised ingestion of about 0.8 ppm protein, 100 ppm vitamin-C and 300ppm calcium per day consumption aids to surmount fluoride toxicity and endemic fluorosis. Calcium gets interacted with fluoride to yield an insoluble salt calcium fluoride being a bulky isn’t absorbed via gastrointestinal tract but excreted faster so calcium rich diet like viz., milk, curd, sardines, cann salmon, beans and lentils, leafy greens/vegetables, cheese and almonds is advisable. Such dietary measures bring down the toxicity of fluoride due to compatible system and available resources [7].
Fluoride remediation or De-fluoridation Methodology
Techniques: Fluoride remediation/de-fluoridation i.e., fluoride removal from contaminated water is achieved as per WHO water quality norms through assorted techniques owing below mentioned enviable characteristics [4-19]:
• Low cost de-fluoridation system needs to be explored
• Easy and facile or handy operations are desirable
• Technique must be free from parameters like key fluoride level, alkalinity, pH, and operating temperature etc.
• Technique must not owe side effects viz aesthetic taste, odor and color of water
• Technique must be reached to the entire rural domain whom is the major sufferers
Extensive R&D on de-fluoridation have given practicable solutions based on diverse principles including coagulation, precipitation, electrochemical, electro-dialysis, reverse osmosis, adsorption, hybrid coalesce adsorption and dialysis are widely chosen. Amid, adsorption methodology is versatile due to numerous grounds like inexpensive, varied end-use applicability, socioapproval, environmentally benign, and dogmatic obedience besides effortless operations. Thus, alternative de-fluoridation methodologies like flocculation, adsorption, co-precipitations, precipitations, coagulations and membrane adsorptions owing enhanced fluoride removal are reported without altering treated water quality, mention below:
a) Membrane filtration: RO filtration membranes are developed to purify several types of water, but reverse osmosis are normally unaffordable due to higher cost RO membranes. The activated alumina based filters are developed for local field usage due to comparatively low cost factors involved in domestic water filtration [17].
b) Distillation: Commercially certain distillation filters are prepared which can remove fluoride and other undesirable contaminations from water.
c) Coagulation: Alum based coagulation found to be successful process for remediation of fluoride water pollutions.
d) Precipitation: Certain chemicals like ion exchangers are developed to carry out precipitation followed membrane separation.
Purpose of de-fluoridation: Remediation of excessive fluoride contamination from water decree success ratio lacking unbiased facts/proofs of the process is achieved via crucial methodical optimization like set-up, socio-economic and environmental conditions ground availability and filed acceptance besides minimum capital/running costs, and water quality are opted for choosing particular process. Thus, de-fluoridation technology which is commonly opted should meet following parameters. Defluoridation process must be simple, reasonable being consistent and functioning at below two levels as domestic/household, institutional/working places and community like village/town [18].
Promising methodologies: Excessive dissolved fluoride can be separated following methodologies as namely precipitation, coagulation, ion-exchange, RO/FO membrane and electrochemical. Existing methodologies also won various drawbacks like less removal capacity, expensive, regeneration problems, waste disposal; field based techno-economic viability etc. Thus, needs to modify certain existing de-fluoridation methodologies in view of above limiting parameters. Distillation, reverse osmosis, electro-dialysis and resin based adsorption eradicate fluoride contaminated water without other its aesthetic parameters. Defluoridation lessens fluoride pollution specially, without further foremost changes in treated water. Such, water de-fluoridation differs from normal water treatments with below reasons:
• Since fluoride not deteriorate water quality not technically and ogano-leptically [17]
• De-fluoridation at large/field level is environmentally unsafe due to respectively accretion of toxic sludge/waste which also generate its proper disposals.
• De-fluoridation is evade if found no problems in transporting low fluoride water pollutions.
i) Sorptive De-fluoridation: Sorption is a physical and chemical phenomenon wherein one substance gets attached on to another substance’s surface. Specific sorption’s are planned like in-plug flow filters, owing elevated capacity absorption. Sorption requires boosting/regenerating medium due to frequent saturations in each cycle of operation. Assorted innate sorption modules are developed through minerals/clays including magnesite, stilbite, goethite, bentonite, serpentin, china clay, fuller’s earth, apophyllite, clinoptilolite and kaolinite. Parameters which affect the sorption phenomenon are capacity, availability and ensuing quality of water limits the utility of such worthwhile materials.
ii) Co-precipitation: Co-precipitation is a kind of precipitation occurs via use of chemicals which gets totally combine with pollutants of water so as to yield huge sludge. Nalgonda techniques grown by NEERI involved primary co-precipitation, coagulation and sedimentation carried means of alum and lime which momentarily bind fluoride of contaminated water [19].
iii) Precipitation: The condensation of materials under gravity performed by means of addition of other chemicals forms assorted states like drizzle, rain, sleet, snow, graupel and hail is called as precipitation. Fluoride form precipitates and simultaneous co-precipitation of calcium and phosphate composites from contaminated water by means of aluminium sulfate and lime treatment. While these precipitation techniques are applied for moderate residual fluoride concentration like 1 to 1.5 ppm scale. Alum/aluminium salts based precipitation methods own several drawbacks viz; moderate, not applied for high fluoride level, create hige sludge, need large dosage of precipitating agent which owe adverse effects in its treatment techniques. Certain precipitation based on lime, calcium, and irons are also administrate low salt solubility thus can’t perform complete defluoridation due to meagre reconcile features of precipitates. Currently grown technique called electrolytic precipitation is also used to removal excess fluoride from contaminated water. Here, fluoride adsorption occurs via precipitating dissolution of aluminium at anode in electrochemical cell that yields Al(OH)3 using alum as precipitating agent for fluoride contaminated water. The electrochemical cell designing with respect to water contamination and % fluoride level are crucial and vital factors for its commercial success. Rather suitable due to relatively lower cost than the conventional precipitation techniques, yet need R&D so as to develop effective electrochemical electrodes viable for potential electrolytic precipitation [20].
iv) Contact Precipitation: Contact precipitation is nothing but precipitation achieved through mixed component salts like calcium and phosphate confide in contact with charcoal getting saturated with pollutants carried out in jar, buckets and fed columns. The contact precipitation is viably govern with efficient crystal growth and catalysed precipitation phenomenon. Fill, mix and filter based appropriate setup are intended which must be inexpensive, corrosion-resistant, robust, high efficiencies, without break down/saturation. Contact precipitation involved calcium and phosphate based precipitating chemicals, operated catalytically onto saturated charcoal surface. Contact precipitation is basically co-precipitation, nonetheless arbitrary is consistent. Contact precipitation process own deprived fluoride removal capacity still preferred due to less troublesome and facile operations. Contact precipitation appears promising, environmentally safe, societal suitable, economic, efficient, consistent and operated at ambient conditions. But its implementing challenges needs to be considered for its user based motivational utility and chemicals accessibility [4].
v) Coagulation and flocculation: Coagulation is an instant process wherein phase change occurs from liquid to gel forming clotting coalesces called coagulates. Coagulation system entails activation, adhesion and aggregation of deposited pollutants. Coagulation begins sub-space initiative processes called changes phase change and deposition of exposed component viable for clotting. Coagulation is chemical charge neutralization whereas flocculation is physical non neutralized phenomenon wherein colloids expel through suspension as a floc/flake achieved via spontaneously or by means of clarifying agent. Coagulation and flocculation are carried out by addition of coagulants/ flocculants clustering small and destabilized pollutant species which form facile separated aggregates from contaminated water. Coagulation-flocculation is preliminary or intermediary in filtration and sedimentation process of water treatments and usage of lime along with bleaching powder in evitable in such water treatment. Both the processes involved methodical stirring and vigorous swirling can thrust further coagulation/flocculation of chemical components resulted heavier flocs/flakes which gets easily settled down at bottom. Flocculation is excellent if carried out under alkaline pH. Metallic salts of iron, aluminium titanium and zirconium are widely used coagulants used in combination with lime and alum yielding insoluble calcium fluoride at highly basic pH owing highly effective coagulation and precipitation of excess fluoride from contaminated water. Initially precipitation occurs through lime treatments followed coagulation as carried by commonly used coagulating agent alum. Alum induces alkalinity that yields aluminium hydroxide [Al(OH)3] which is non-soluble in water and carried out precipitation besides aluminium reaction with residual fluoride of contaminated water. Coagulation removes minor portion of fluoride as a valuable precipitate while converts large portion as soluble aluminium fluoride complex and toxic thus and not desirable. Aluminium sulfate coagulant induces surplus sulfate adulteration in treated water causes therapeutic adverse effects. Residual aluminium level in treated water is viable for many disorders like musculoskeletal, respiratory, cardiovascular, endocrine and reproductive systems besides structural, biochemical changes like dementia and neuron dysfunction. Nalgonda technique uses hydrate aluminium salts for coagulating and flocculating residual excess fluoride from contaminated water [4-6].
vi) Electro-coagulation: Coagulation achieved through an electrochemical phenomenon called electro-coagulation as occurred onto aluminium electrodes. Electro-coagulation is resulted water de-fluoridation onto aluminium bipolar electrodes owing aluminium-fluoride mass ratio of 17:1. Basically this process involved adsorption/deposition of fluoride anions through aluminium hydroxide precipitation and anodic dissolution of aluminium in a given electrochemical cell. The electro-coagulation process uses high electricity of 0.3 to 0.6kwh per thousand litres of water which is costly. Besides anode materials gets constantly consumed thus wants replenished. This electro-coagulation also creates huge solid-sludge/wastes on dry basis [4].
vii) Membrane based De-fluoridation: Membrane is thin pliable sheet/layer of material creating acts as a boundary/lining/ partition in separating two components. Mitigation of excess fluoride from water is also achieved through assorted membrane based processes including reverse/forward osmosis (RO/ FO), nano-filtration and electro-dialysis. Although Membrane derived water de-fluoridation are unconventional purification, non-user-friendly and expensive techniques, yet RO membrane process are favoured. Reverse osmosis is physical phenomenon achieved by pressure driven through semi-permeable membrane at its concentrated side (defeats innate osmotic pressure). Membrane discards corresponding ions as per size and electrical charges. Many factors persuade membrane based water defluoridation process viz; its selection, cost, recovery, rejection, water characteristics and pre-treatment besides pressure, temperature and regular monitoring. RO/FO defluoridation own some limitations like develops fouling, induces microbiological growth, yield intense brine discharge, significant water loss being unsuitable at large scale. Electro-dialysis is also a membrane carried separation process akin RO but uses direct electric current (instead of pressure) in separating residual pollutants from raw water. However, electro-dialysis carried defluoridation is disadvantages over RO membrane purification due to high energy intensive factors as unsuitable for large scale utility. Reverse osmosis is the physical phenomenon wherein components get separated directly via pressure applied by semi-permeable membrane at its concentrated which is reverse to natural osmosis due to defeating innate pressure. Reverse osmosis membrane found to reject residual pollutant ions based on its size and electrical charge. Certain factors influencing this RO/FO based de-fluoridation like membrane selection are cost, recovery, rejection, raw water characteristics, pre-treatment pressure, temperature, normal monitoring and maintenance. Membranes are sued to eradicate fluoride from contaminated water includes nano-filtration operating comparatively at low pressure and reverse osmosis RO function at huge pressures owing superior refutation of all suspended solids. Maximum fluoride removal capacity is reported by NF, RO membrane based water purifications. Assorted progressive technical developments are done in designing materials for NF/RO/FO based membrane de-fluoridations being economic, competitive and extremely consistent. These membrane derived de-fluoridation are beneficial than other conventional treatment methodologies [4-6]. Although RO/FO, NF based membrane de-fluoridation appeared alternative to conventional methodologies, yet own limitations viz; expensive, residual acidity in water requires balancing pH to neutral conditions for drinking purpose, wasted huge water as brine so needs disposal as achieved via minerals addition or re-mineralization after water treatment [21].
viii) Ion Exchanger based De-fluoridation: The exchange of ions amongst two electrolytes and/or electrolytic solution-complex is achieved through functionalized porous/gel polymers called resins. Many materials are also used to perform ion exchange phenomenon like zeolites, montmorillonite, clay, and humus- soil. Basically ion exchanger exists as cationic replacing cations and anionic replacing anions from sample besides amphoteric nature which exchange of cations and anions simultaneously. Most of the ion exchangers are unselective or have binding preferences for certain ions or classes of ions based on innate chemical structures. Ion exchange phenomenon found to be dependent on size/shape of ions and their charges besides their structure. Attributed ions found specifically bind to solid polymeric / mineralic “ion exchange-sites” via separation/de-minerization of ion-containing solutions.
Certain cation exchange resin like saw dust carbon based Defluoron–1, Carbion and Wasoresin-14 besides polystyrene are commonly used for fluoride removal from water. Mostly used anionic exchange resin includes Tulsion-A-27, Lewatit-MIH-59, Amberlite, IRA-400 and deaceodite,FF-1P are preferred for defluoridation of water [4-7]. Anion exchange resins own some drawbacks over cation exchangers like comparatively low removal efficiency, unusual taste to treated water and, non-economic for large scale de-fluoridation thus unacceptable. But, strong-base anion resins own adsorptive capacity for water dissolved fluoride anions due to slower film diffusion phenomenon quite different to intra-particle diffusion. Tough base exchanger resins adsorbs fluorides onto specially own hydroxyl or chloride proactive sites from contaminated water. Certain inorganic ion exchangers are developed for this purpose like metal chloride silicates, barium/ ferric chloride silicic acid based ion exchange complexes used for removal of fluoride from water. Cationic exchanger resins like ‘Avaram Bark’ blended with alum appears to be good defluoridating agents for high fluoride contaminated water.
Polystyrene based anion exchange resins owing strong basic quaternary ammonium linkages effectively eliminate fluorides from water. The activated alumina, activated charcoal, and ion exchange resins are most efficient in mitigation of high fluoride contaminated water/wastewater. Adsorptive ion exchangers can capture fluoride anions from one phase and condensate/ concentrate on another phase’s surfaces. Ion exchange based de-fluoridation observed at specially and rationally designed boundary phases of liquid- liquid, gas-solid, gas-liquid, liquidsolid surfaces [22,23]. Assorted adsorptive de-fluoridation methodologies based on physical and chemical adsorption besides ion exchange phenomenon are mentioned in below Table 6 [4-6].
Table 6. Assorted adsorptive de-fluoridation materials for De-fluoridation.
Physical, chemical and exchange adsorption techniques involved electrostatic binding of fluoride anions onto active sites of adsorbent surfaces. These adsorption are instantaneous assign in solitary step govern by surface activity, rate and extensive surface area of adsorbents used in such process of de-fluoridation. The varied adsorbent materials are developed via calcium salts like Ca (OH)2, CaSO4 and CaCl2 for carrying effective de-fluoridations of industrial fluoride bearing wastewater although enhances calcium content in the treated water. Fluoride adsorptive mitigation is achieved through bone charcoal, as bone surfaces own huge surface area besides carbonate-apatite embraced in bone as Ca (PO4)6-CaCO3 are viable for investigative fluoride anionic exchange and yield insoluble fluorapatite [Ca(PO4)6.CaF2] onto bone surface viable for investigative de-fluoridation of water [17-23].
The bone carbonizing at 1100°C yields bone char materials which shown superior ion exchange capacity for residual fluoride adsorption from water than mere unprocessed bone. Activated alumina owing aluminium oxide being amphoteric bind fluoride anions onto its active surfaces at altering conditions and forms [(Al2O3)n AlOH3]complex which is easily separable. Aluminium complex formation is firmly depends on acidity and alkalinity of residual sample water and thus its extensively used adsorbent for drinking water purification. Alumina caused high fluoride adsorption found reliant upon certain factors including innate crystallinality, pH, acidity and alkalinity. The γ-alumina is observed to be ten times efficient than α-alumina for fluoride at limiting acidic pH. Furthermore, alumina starts leaching at strongly acidic conditions and creates threats to health due to aluminium induced poisoning like Alzheimer’s disease.
ix) Nalgonda process of Defluoridation: The comprehensive R&D of CSIR-NEERI laboratory have developed easy operable/handling and inexpensive defluoridation process called Nalgonda technique for fluoride contaminated water. Nalgonda is named after the town wherein method as initiated/adopted in the year 1965-70 on large scale for getting pure potable water at community level. This technique uses alum and lime doses empirically 1/20th of alum (varied with fluoride ratio and alkalinity) as coagulating and flocculating agent respectively along with bleaching powder as disinfectants for de-fluoridation of fluoride contaminated water. High fluoride content in raw water creates huge solid-waste/ sludge which needs proper treatments. Nalgonda technique involved rapid mixing, flocculation, sedimentation and filtration induced de-fluoridation used for domestic and community level water treatments effective for more than 20 ppm fluoride contaminations [4,24]. Nalgonda’s efficacy conveyed its wide range applicability though enough alkalinity in raw water being essential to attain upmost de-fluoridation capacity. More usage of alum dose induces residual aluminium leakage in treated which are viable for neuro-toxicity restrict its utility further. De-fluoridated/contaminated water sample must own TDS < 1500 ppm and desalination is essential if total dissolved solids > 1500 ppm [25]. Effective de-fluoridation is viable when total hardness of sample water is < 250 ppm [5,23-25]. De-fluoridation via Nalgonda technique needs water sample to be analysed for initial fluoride amount, alkalinity, TDS and hardness identified before assessment and fixing alum-lime dosage besides routine operational monitoring [6,26].
Regulations and advisories for fluoride controls: An international regulations and advisory guidelines for fluoride controls in air, water and other media are mentioned like chronic level of oral fluoride/kg per day is 0.05 mg [4]. The Maximum Contamination Limit (MCL) is 0.15 mg fluoride/kg per day, but 0.25 mg fluoride/ kg/day induces skeletal effects and large fractures [26]. MCL of 0.02 ppm fluoride is allowed for HF usages in industrial process/ products and MCL of 0.5 ppm for upper respiratory tract inflammation in humans. Acute inhaled MCL of above 0.01 ppm fluorine gas causes respiratory irritation in man [5]. Environmental Protection Agency (EPA) has derived an oral Reference Dose (RfD) of 0.06 mg/kg/day for soluble fluorides in diet, water and MCL of 0.12 mg/kg/day for dental fluorosis in child [6].
Remedial/preventive in Fluoride toxicity: Various curatives/ preventives are adopted to alleviate endemic to fluorosis in sufferer. These comprise following measures:
• To provide option of water sources owing low/safe fluoride level as per World Health Organization guidelines (WHO, 2006)
• To give safe drinking water though surface/ground water supply or
• To implement rainwater harvesting structures in recharging of groundwater, too aids dilution of fluoride in groundwater/ drinking-water to an acceptable limit
• To blend fluoridated water with good quality water is quite effective
• To supply nutritional diet as counter to detrimental effects of excessive fluoride consumptions like ample Vitamin-C combats fluoride adversity and reduce dental fluorosis
• To furnish dietary Ca++ which interacts with fluoride to form insoluble CaF2 can be excreted easily by excretion system
• To implement effective de-fluoridation techniques like Nalgonda techniques, Bio-F process, precipitation, flocculation, sedimentation, filtration, disinfection coagulation, adsorption- based methodologies, ion exchange and membrane based RO separations
• To expand community-based defluoridation unit as costeffective, easy technological operations but keeps below fluoride permissible limit
• To identify pertinent gaps in offered reasonable ongoing appropriate defluoridation techniques and prospective scientific interventions
• To develop workable strategies to offer fluoride-safe water to rural and affected areas
• To popularize varied cheap and simple de-fluoridation methodologies as already famous, but benefits have not reached the rural or affected population due to any limitations
All the above mentioned remedial/preventives are vital in mitigation of fluoride toxicity in groundwater and human exposure besides viable health hazardous besides. Therefore, choice of the used de-fluoridation process/technique is an important consideration.
Conclusion
High concentrations of fluoride in groundwater arise due to minerals/ores in rocks/sediments besides synthetic actions are key toxicological and environmental issues. The conditions like weathering, leaching and industrialization are pivotal in contributing fluoride contaminations. Excess fluoride consumptions induce varied health hazardous depending on its exposure, body-weight, water/food intake and climate. Many simple and effective defluoridation techniques are known while formulation of strategy is required for providing fluoride-free water. The fluoride pollution is a major environmental disaster being faced by almost every country on this planet. Thus it’s inevitable to explore viable causes of fluoride contaminations in every part of environment and to find remedies for fluoride pollution. This is an overview of fluoride: toxicological profile, pollution aspects, remedial measures and treatment methodologies beside preventive step for mitigation of fluoride pollution.
Acknowledgements
Author is thankful to Head, Department of chemistry, R.T.M. Nagpur University, Nagpur, for providing the facilities needed to carry out this research study and also thankful to Director, VNIT Nagpur, and Director, NEERI, Nagpur MS, for providing the water sample analysis. Author is also thankful to the Vice Chancellor, R.T.M. Nagpur University, Nagpur, India for sanction of University Research Project Scheme vide letter, No. Dev/ RTMNURP/ AH/1672 (9) dated 24 September 2016.
References
- Water for Life: Making it Happen (2005) World Health Organization (WHO) & UNICEF report.
- Pearce F (2006) When the rivers run dry: journeys into the heart of the world's water crisis. Toronto.
- UNICEF (1999) States of the Art Report on the Extent of Fluoride in Drinking Water and the Resulting Endemicity in India. Report by Fluorosis and Rural Development Foundation for UNICEF, New Delhi.
- Dongre RS, Ghugal DN, Meshram JS, Ramteke DS (2012) Fluoride removal from water by zirconium (IV) doped chitosan bio-composite. African J Environ Sci Technol 6: 130-141.
- Susheela AK (2007) A treatise on fluorosis: Book, 3rd Ed. by Fluorosis Research & Rural Development Foundation.
- Susheela AK (1987) Fluorosis in India, the magnitude and severity of the problem. Sci Dev Env pp: 147-157.
- WHO (1997) Guideline for Drinking Water Quality Health Criteria and Other Supporting Information, 2nd ed. Geneva 2: 122.
- Susheela AK (1999) Fluorosis management programme in India. Current Sci 77: 1250-1256.
- Risk Assessment Guidance for Superfund Volume I: Human Health Evaluation Manual; U.S. Environmental Protection Agency, Office of Emergency and Remedial Reponse: Washington, DC.
- Singh B, Prasad M, Amritphale SS (2004) Development of Defluoridation Technology for its Easy Adaptation in Rural Areas. J Rural Tech.
- Rajiv Gandhi National Drinking Water Mission (RGNDWM) (1993) Prevention and control of fluorosis in India, Health Aspects. Ministry of Rural Development.
- Indian Council of Medical Research (ICMR) (1975) New dimensions to fluorosis in Andhra Pradesh 5: 1-5.
- Choubisa SL (2001) Endemic fluorosis in southern Rajasthan, India. Fluoride 34: 61-70.
- Misra AK, Mishra A, Premraj (2006) Escalation of groundwater fluoride in the Ganga alluvial plains of India. Fluoride 39: 35-38.
- Das B, Talukdar J, Dutta RK, Das SC (2003) Fluoride and other inorganic constituents in ground water of Guwahati, Assam. Current Sci 85: 657-661.
- Sengupta SR, Pal B (1937) Iodine and fluoride contents of food stuffs. Ind J Nutr Dicter 8, 66-71.
- Lakdawala DR, Punekar BD (1687) Fluoride content of water and commonly consumed foods in Bombay and a study of dietary intake. Ind J Med Res 16: 1679-1687.
- Meenakshi S, Viswanathan N (2009) Defluoridation of water using magnesia/chitosan composite. J Hazard Mater 162: 920-930.
- Dongre RS (2018) Biosorption of Fluoride from Water by Fabricated Chitosan Doped Graphite Novel Composite. Research Development in Material Science 1: 1-9.
- Susheela AK (2003) A Treatise on Fluorosis, Revised 2nd ed., Fluorosis Research and Rural Development Foundation, New Delhi, India.
- Dongre RS (2018) Rationally Fabricated Nanomaterials for Desalination and Water Purification. Novel Nano 1: 348-366S.
- Dongre RS (2018) Chitosan-Derived Synthetic Ion Exchangers: Characteristics & Applications. In-Tech Open, Croatia 1: 21-42.
- Dongre RS (2020) Nanomaterials via Reconfiguration of Skeletal Matrix. Nanostructures pp: 181-206.
- Dongre RS (2020) Reinforce Fabricated Nano-Composite Matrixes for Modernization of S & T in New Millennium in book Composite & Nanocomposite Materials From Knowledge to Industrial Applications. Pp: 38-84.
- Sengupta P, Saha S, Banerjee S, Dey A, Sarkar P (2020) Removal of fluoride ion from drinking water by a new Fe(OH)3/ nano CaO impregnated chitosan composite adsorbent. Polym-Plast Technol 29: 1191-1203.
- Dongre RS (2019) Chitosan Formulations: Chemistry, Characteristics and Contextual Adsorption in Unambiguous Modernization of S&T. In book, Hysteresis of Composites pp: 469-491.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences
