Factors Affecting the Procurement of Pharmaceutical Drugs: A Case Study of Narok County Referral Hospital, Kenya
Joy Muhia, Lynn Waithera and Rogers Songole
DOI10.21767/2471-299X.1000061
Joy Muhia1, Lynn Waithera1 and Rogers Songole2*
1Member of Young Professional Chronic Disease Network (YPCDN), Beyond Science Initiative (BSI), Kenya
2Department of Mental Health, MOI University, Kenya
- *Corresponding Author:
- Rogers Songole, PhD
Clinical Psychology, Senior Lecturer in Mental Health
MOI University, Kenya
Tel: +254721208676
E-mail: rogerssongole@gmail.com
Received date: November 24, 2017; Accepted date: December 08, 2017; Published date: December 18, 2017
Citation: Muhia J, Waithera L, Songole R (2017) Factors Affecting the Procurement of Pharmaceutical Drugs: A Case Study of Narok County Referral Hospital, Kenya. Med Clin Rev. 3:20. doi: 10.21767/2471-299X.1000061
Copyright: © 2017 Muhia J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Procurement of pharmaceutical drugs plays a crucial role in management of health. It was observed there was lack of some drugs in Narok County Referral Hospital (NCRH) and it caused a strain on the patients. The patients have to buy the prescribed medication from chemists and pharmacies outside the hospital which is very expensive. The availability of drugs is crucial in the functioning of any hospital. For there to be continuity of flow of drugs, their procurement procedure is essential and objectives should be considered in the procurement of pharmaceutical drugs. The objective of this study was to determine the factors affecting the procurement of pharmaceutical drugs in Narok County Referral Hospital. Descriptive crosssectional study was carried out at NCRH. Key personnel strategically positioned to integrate with the procurement process of pharmaceutical drugs were included in the study. Data was collected using semi-structured interviews and later transcribed to notes. Themes were deductively analysed before being presented in prose. The study revealed that the procurement procedures in NCRH followed the recommended guidelines. Major challenges included bureaucracy, poor quantification, inadequate transportation options and lack of skilled personnel. The main factors facing the procurement of drugs in NCRH were inadequate funding resulting in delay in paying the suppliers and poor quantification. The study recommended that checks and measures should be included, thorough auditing to avoid misappropriation of funds and provide accountability and transparency. Inventory management training by government is needed to equip the staff with knowledge and skills on use and management of the various registers and forms for proper quantification.
Keywords
Procurement; Factors; Pharmaceutical drugs; Accountability; Quantification
Introduction
Procurement is the acquisition of goods and/or services at the best possible total cost of ownership, in the right quality and quantity, at the right time, in the right place and from the right source for the direct benefit or use of corporations, individuals, or even governments [1]. Pharmaceutical drugs play a critical role in the management of health as whenever one is sick drugs are always prescribed by doctors. This makes their purchase to represent one of the largest shares of health expenditure in any country worldwide ranging from 5% to 12% in developed countries to as much as 40% in developing countries [2]. The availability of drugs is thus crucial in the functioning of any hospital. For there to be continuity of flow of drugs, their procurement procedure and challenges faced are essential and objectives should be considered in the procurement of drugs. Some of these objectives include quality, price, speed and ethics. When correct procurement procedures are followed, it helps to curb some of the avoidable problems that may be experienced like overstocking or under stocking of drugs.
In Kenya, before devolution, procurement of drugs was handled at a national level where all the drugs were procured nationally as all hospitals would send their requisitions to the Ministry of Health and they would then be distributed to the various hospitals nationwide and the settlement of funds to the supplier was done by the national government. After devolution, this is the sole responsibility of the county governments where the money is distributed to the various counties which are responsible for the running of all services in that county such as health. As a result some counties may have adequate drugs while others experience shortages at different times depending on the county management.
Procurement process is part of the management cycle of drugs. This goes hand in hand with selection of drugs, quantification of drug needs, storage and distribution/supply.
Statement of the problem
The researcher observed that whenever drugs were prescribed they were followed with a directive to either proceed to the hospital pharmacy or with an apology by the healthcare provider that the prescribed medication was currently unavailable at the hospital pharmacy and that they had to buy them from a chemist. This was at times met with a blank stare or the patient asking for an alternative medication. Other times they asked if the medication could wait so that they could go and source for funds. Having noted this lack of some drugs and the strain it caused on the patients, it was found necessary to find out some of the factors in the management cycle of drugs especially procurement that would contribute to this problem.
Justification
During attachment period at NCRH the researcher observed that there was lack of some drugs in the hospital and the patients were therefore forced to buy the prescribed medication from chemists and pharmacies outside the hospital. This was very expensive and most locals could not afford to buy the medication. This study aimed to identify the factors leading to this lack of drugs and how to avoid this situation in future. An effective procurement of drugs and tackling of the challenges faced is critical in the normal functioning of any hospital facility as it will lead to better management of health and at an affordable rate.
Research Objectives
Broad objective
To determine the factors affecting the procurement of pharmaceutical drugs at NCRH.
Specific objectives
To determine the procurement procedure at NRCH.
To determine the challenges facing the procurement of pharmaceutical drugs at NRCH.
Literature Review
Introduction
Procurement is an important part of efficient drug management and supply and is an important procedure for all levels of health care institutions. An effective procurement process ensures the availability of the right drugs in the right quantities, available at the right time, for the right patient, at reasonable prices, and at recognizable standards of quality. Thus, procurement is not simply the act of buying but encompasses a complex range of operational, business, information technology, safety and risk management, and legal systems, all designed to address an institution’s needs. Specifically, individuals involved in pharmaceutical procurement must determine, accredit, and monitor appropriate supply sources; evaluate suppliers’ performance; choose a buying strategy or approach; monitor drug delivery; assess clinical and use outcomes; and evaluate new products and the drug market. Successful hospital procurement is also a collaborative process, involving people with skills in purchasing, finance, management, clinical and nursing specialties, pharmacy, quality control, and even the end user: the patient [2].
Procurement procedure
The supply and management of drugs is a continuous cycle. The drug management cycle includes the selection of drugs, quantification of drug needs, procurement, storage and distribution. The selection of drugs for procurement should be based on the national essential drugs list. Quantification is necessary to avoid wastage through over-stocking or stockouts of pharmaceuticals. Procurement through competitive tenders aims to provide quality drugs at the lowest possible cost when needed. The procurement system is in compliance with the requirements of the Government Procurement Agreement of the World Trade Organization [3]. Correct storage of drugs is essential to avoid deterioration and waste. It also includes the use of drugs, quality assurance and monitoring and improving the drug management cycle [4].
Given the impact of procurement activities on the operation and effectiveness of a hospital, it is essential that these activities be performed by qualified staff with high professional and ethical standards and using sound procedures anchored in appropriate policies and regulations. Pharmacists involved in hospital procurement of medicines, whether directly or indirectly, must be knowledgeable about medicines as well as the interacting issues and the many stakeholders who can potentially affect the process or who may have legal responsibility [2].
Key areas of attention include transparency, cost containment, technical capability, operational principles of good pharmaceutical procurement, purchasing for safety, ensuring appropriate selection, timely, accurate and accessible information, ensuring quality products, and proper budgeting and financing [2].
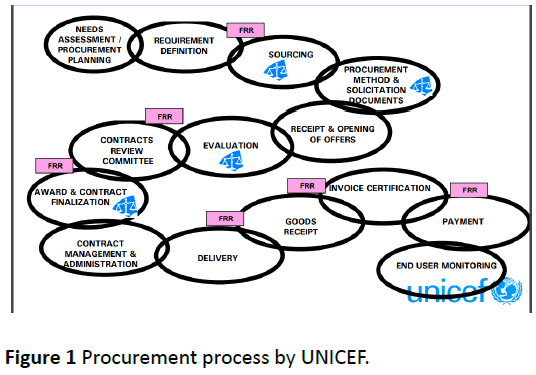
Procurement process is governed by UNICEF Financial Rules and Regulations and includes appropriate segregation of duties (Figure 1) [5].
The Government of Kenya procures medicines mainly through Kenya Medical Supplies Agency (KEMSA). It has been estimated that KEMSA’s purchases constitute 30 per cent of all prescription drugs in the Kenyan market. The Agency also procures for some donor partners. KEMSA’s 2010/2011 Government budget (not counting donor contributions) for the procurement of essential medicines for public hospitals is US$ 19.8 million; together 3 Executive Summary with US$ 29.7 million for Rural Health Facilities (Dispensary Kits + Health Centre Kits + Loose Drugs RHF), this makes a total of US$ 49.5 million. Out of 343 items on the Essential Drug List (EDL), KEMSA procures only about 117 selected items, based on available funds. Many EDL medicines cannot be purchased because of budgetary constraints. Mission for Essential Drugs and Supplies (MEDS) is another large-scale, bulk procurer of medicines.
It is a not-for-profit organization which procures medical items for Faith-Based Organizations (FBO) and some donors. About 45 per cent of MEDS’ annual turnover of 800 million to 1 billion KSh (about US$ 12 million) is spent on medicines. Some donors procure directly. Purchases from Global Fund donations are undertaken by the Procurement and Supply Chain Management Consortium, which is made up of four partners, KEMSA, Crown Agents, Deutsche Gesellschaft fur Technische Zusammenarbeit (GTZ), and JSI - John Snow Inc. [6].
Challenges faced
Globalization has made the pharmaceutical industry procurement and supply chain more complex than ever before. Today, raw materials are often sourced in one country, processed in another, with the finished product being sold in a third. This complicated supply chain has only increased the importance of track and trace technology on pharmaceutical processing lines to enable manufacturers to demonstrate compliance with relevant safety regulations throughout its operations [7]. Common procurement challenges include inadequate rules, government policies regulations for procurement and institutional structures which sometimes hinder overall efficiency in responding to the modern pharmaceutical market. Public sector staff with little experience in responding to market situations as Market constraints differs from country to country. Public sector drug procurement must take place in the context of both the local pharmaceutical market and the international market. Absence of a comprehensive procurement policy such as if there is an appearance of special influence on the selection of products and suppliers or if the procurement process is not managed in an efficient and transparent manner, interest among suppliers in competing for procurement contracts decreases, leading to fewer choices and higher prices for drugs. Limited or irregular funding which leads to delays in payments worsens procurement problems as suppliers deny credit or insist on advance payments. A degree of financial autonomy for the health system, while providing flexibility, requires proper accountability and efficient management [8].
Donor agencies with conflicting procurement regulations; which in turn may conflict with existing local laws and regulations. In these situations it is extremely difficult to carry out procurement in a timely and efficient manner. Development assistance should be more consistent with the policies of the country. And it is essential that this assistance should reinforce good pharmaceutical procurement practices and aim at sustainability, rather than undermining or delaying the development of such procurement practices. Contracting out parts of the procurement/distribution function may improve efficiency and reduce costs. But this will only be true if public health systems can properly monitor and manage such contracts. In many countries the necessary experience and information systems for this are lacking. In some countries initial decentralization of drug procurement was followed by pooled procurement by hospitals or cooperatives. Unbiased market information on product availability, comparative pricing, product quality and supplier performance is difficult to obtain in many countries. Poor access to information is most common in countries where it is most needed in the light of inadequate regulation of the local market. This information deficiency can result in gaps in essential drug availability and in procurement of poor-quality products at unnecessarily high prices. It may also facilitate undue influence on the procurement process by special interest groups. Lack of trained procurement staff in key positions can doom any procurement system to failure. While effective training programs can remedy this problem, in many supply systems there is limited access to training in good procurement practices. Also unattractive public sector salaries and lack of career development tend to restrict capacity to attract and retain qualified staff [8,9].
Developing countries have had difficulties in maintaining public supply systems, due to frequent breakdowns that regularly occur at multiple points in the process. Many public procurement activities suffer from neglect, lack of direction, poor co-ordination, lack of open competition and transparency, differing levels of corruption and most importantly not having a cadre of trained and qualified procurement specialists, who are competent to conduct and manage such procurement and distributions, in a professional, timely and cost effective manner. Inflexible and bureaucratic procurement contribute to unacceptable contract delays, increased costs, the potential for manipulation of contract awards and lack of fair competition, all of which create the perception in the population at large, that public expenditure is slow, ineffective, expensive and often corrupt [10].
Methodology
Study design
The study adopted a descriptive cross-sectional research design. This is because the research was observational at a specific point in time, across all the relevant strata of the hospital staff. This design was deemed suitable since it enabled collection of immense data within limited time.
Study site
The study was conducted at Narok County and Referral Hospital.
Background of study site
Narok County Referral Hospital (NCRH) is located in Narok North County. Narok is located in Kenya 109 km from Nairobi at the longitude of 35.85 and latitude of -1.07. The area’s temperature range is 12 to 28°C and the average rainfall range of 500 to 800 mm per annum. The county covers an area of 17,933.1 km and is classified by an agro-pastoral area. NCRH was previously a district hospital but with devolution the hospital has been upgraded to a county referral Hospital. It serves a population of approximately 850,920 people.
Study population
The key informants were the Medical Superintendent, Pharmacy officer in charge, 2 procurement officers, 3 pharmacists, the pharmacy storekeeper and 2 store and supplies staff.
Sampling technique
Purposive sampling was used in this study.
Eligibility Criteria
Inclusion criteria
Those who were better positioned to interact with the pharmacy procurement procedures were included as respondents in the study.
The key informants were selected for their ability to provide relevant information concerning the study.
Data Collection Procedure
Data was collected using semi-structured interviews and transcribed to notes. The interviewing only stopped when theme saturation was attained. Data was recorded by note taking during the interviews.
Data Analysis Procedure
Thematic analysis was done whereby themes were deduced from the transcribed data guided by the specific objectives. Coding of recurrent themes was done to make referencing easier.
Ethical Considerations
Approval to conduct this study was sought from Moi University Institutional Research and Ethics Committee (IREC). Permission to conduct the research within the hospital was requested from the medical superintendent and carried out thereafter. Each of the interviewed participants gave verbal consent prior to the start of each interview session. The anonymity and confidentiality of the respondents was maintained throughout the study. Only the researcher had access to the transcripts of the interviews.
Findings
Procurement procedure
The drug management cycle consists of selection, Procurement, Distribution, and Use.
The process of selection of drugs
The study found that drugs were ordered with generic names and the selection of drugs for procurement was based on the national essential drugs list. Supply was mainly from KEMSA. Tender was based at county level. The hospital’s pharmaceutical needs were compared with the KEMSA list of drugs that they had. Their filling rate was approximately 80%. Out of 343 items on the Essential Drug List (EDL), KEMSA procured only about 117 selected items, based on available funds. If KEMSA did not have a particular drug, they would commit to bring the drugs after two weeks. At times if KEMSA did not have a particular drug, it could be procured from MEDS. There were other minor suppliers identified by the county government to supplement.
Quantification of drug needs
Monthly report on consumption of drugs was normally written from data that was compared with the stock card which helped indicate which drugs were running out. They helped to identify the quantity of different drugs that were used in the hospital monthly. This was then used to procure drugs. The pharmacist officer in charge made a requisition of the drugs that were required.
Procurement
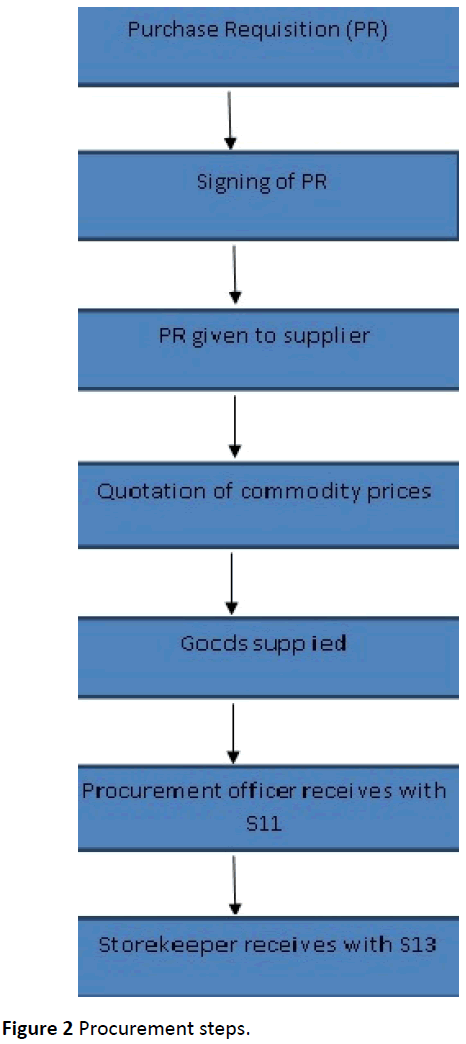
The Process involved the purchase requisition being taken to the procurement office. The requisition was signed by four people, the head of department, the head of procurement, medical superintendent and the Authority to Incur Expenditure (AIE) holder. The requisition was written in quadruplet whereby the original copy remained with the head of procurement and the copies were given to the supplier and store (Figure 2).
Payment process
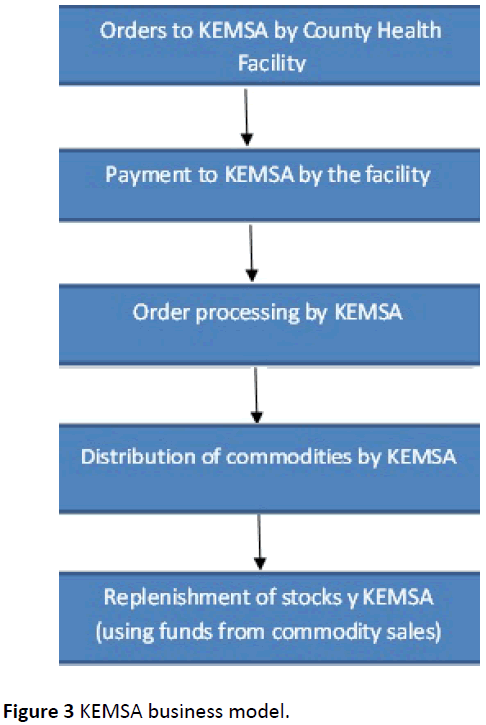
In the old model, once the supplier brought the goods, the payment process then began whereby the local purchase order (LPO) and S13 form were attached in a file and taken to the county finance office. The documents, which were the Invoice, S13 and LPO, were cross checked to confirm the order. They were then put in the system and the supplier was paid. KEMSA was a parastatal before devolution, thus once drugs were procured and delivered, the follow- up of payments was done at the national level. In the new model, after devolution of health, funds were allocated to the various counties who then paid the suppliers. Their payment was thus determined by the management of the allocated funds by the various counties, in the new model (Figure 3).
Storage and Distribution
KEMSA transported the pharmaceutical drugs to the various hospitals and dispensaries in different locations nationally. Once the drugs arrived at the hospital, they are received by the procurement officer who forwards them to the pharmacy store. From here, some like vaccines are distributed to the various hospital departments such as the ante-natal and postnatal departments and this was recorded in an S11 form. For storage, a stock card (S3 and S5 forms) are used to show the various drugs used from the store and the quantities that were still available.
Use
Drugs from the NCRH pharmacy were given as prescription only medication (POM). Any medication given to patients was recorded in a daily activity register and Facility Consumption Data Report and Request Form (F-CDRR), from which a monthly report was written. This was used to inform the selection process by reporting what was currently in the facility and what was not there.
This was a repetitive cycle and the process took averagely three months. At times it depended on payments made because if paid promptly they would be delivered. Ideally the supply period for a dispensary should have been three months while that of a hospital should have been two months. Drugs were ordered for the whole county thus the NCRH ordered drugs for themselves and health centres and dispensaries around. On delivery, these drugs would be transported to the various health centres and dispensaries by KEMSA.
Challenges in the Procurement Process
The challenges faced in the procurement of drugs included:
Inadequate funds
In the payment process, the county director of health received the LPO and crosschecked the order then paid the supplier. When a quotation was done, it was crosschecked at the county level with the amount of money available. If it was more than what was there, some changes were made to the list to suit the funds. Insufficiency of funds would be resulting from corruption whereby the money did not get to meet its target need as allocated. With lack of funds, came a delay in the payment of the supplier and this resulted in lack of trust and eventually the supplier would refuse to supply the goods unless they were paid.
Bureaucracy
Bureaucracy tended to have a negative aspect which became a problem as different counties involved different procedures in the procurement process. Government financial regulations required that no expenditure would be incurred without authority to Incur Expenditure. Thus at times the hospital would have to wait for the relevant signatures before spending on purchases. This resulted in unacceptable contract delays, a delay of payment to the suppliers, increased costs and the potential for manipulation of contract awards and lack of fair competition.
Poor quantification
The hospital had its own quantification based on their daily use and monthly reports made. This helped to know the number of patients treated in a given month and the amount of drugs dispensed for that population. Poor quantification of drugs led to an overestimation or underestimation of the drugs needed thus resulting in over ordering or under ordering of drugs. In the case of over-ordering, it brought about the challenge of drugs expiring before they had been prescribed and this resulted in wastage of drugs and funds as expired drugs became poisonous and thus useless. In the case of under estimation, it resulted in drugs ending before the population served was reached thus resulting in a shortage of drugs and patients were forced to buy medication.
Transportation of drugs
Drugs ordered from KEMSA or MEDS were normally transported to the various destinations of the various hospitals located nationally. Nonetheless, when drugs were ordered from smaller sources, the issue of transport came in as one had to cater for their transport. This was a delicate matter as some of the drugs required special storage conditions for example, vaccines needed to be refrigerated. Sourcing for transport would then need to incorporate various factors such as distance, as some facilities were also very far, so as not to damage the drugs before they reached their destination, thus one needed to be sure of a reliable transport system. Transportation also posed a challenge where there was poor infrastructure.
Other challenges included in rare cases where KEMSA brought drugs that had not been ordered or brought more of the non-commonly used drugs and less of the normally used drugs. This would happen when KEMSA had an overstock of a specific drug. This would go unnoticed if one was not keen or received the drugs from KEMSA without going through the correct and ordered steps of procurement. It would also occur if the procurement of drugs was handled by someone not trained or skilled in the pharmacy docket. The pharmacy storekeeper was also a pharmacist. Shortage could also occur from discrepancies such as when some of the tablets became crushed to powder, arithmetic errors and lack of recording of data in daily activity register.
Discussion
Procurement procedure for pharmaceutical drugs was similar to that of other goods as there were standard procurement guidelines. The procurement of drugs was done on a tender basis. Nonetheless there was no competitiveness as they always went to majorly one supplier, KEMSA. MEDS was another large-scale, bulk procurer of medicines but it was a not-for-profit organization which procured medical items for Faith-Based Organizations (FBO) and some donors. Studies such as one done by the Food and Health Bureau, 2013 have shown that procurement through competitive tenders aims to provide quality drugs at the lowest possible cost when needed.
NCRH procured drugs for the health centres and dispensaries in the county. This was a positive factor because a single procurement list for a large group of facilities increased volume promoted reduced price and improved market presence which led to better supply security and quality [11].
All the documents used in the procurement process such as form S11, S13, stock card, invoice etc. needed to be orderly arranged into different files for easy reference.
Comparison between the old and new KEMSA business model for procurement Table 1 [12].
| Old model | New model | |
|---|---|---|
| Commodities | Medical commodities and supplies bought by KEMSA with funds provided by Ministry of Health (MOH). | KEMSA responsible for procurement of medical commodities and supplies with its own funds. |
| Commodity Order management | Ordering done quarterly by facilities on a PULL system (demand driven). | Ordering done by counties according to their needs. |
| Payment | MOH reimbursed for distribution costs and paid for warehousing costs. | The county government will meet the cost of distribution and commodities. |
| Stock replenishment | MOH replenished the stocks through procurement by KEMSA. | KEMSA shall replenish its stocks from funds realized from sales of commodities to counties. |
Table 1: KEMSA models.
Misappropriation of funds and budgetary constraints played a crucial role in procurement problems. Without the needed or appropriate funds, drugs could not be acquired leading to inevitable lack in the hospital. Limited or irregular funding which led to delays in payments worsened procurement problems as suppliers denied credit or insisted on advance payments. This is the case as currently, the county owes KEMSA which has resulted in at times delay of drugs supply. This lack of payment to the supplier brought about a lack of trust. This resulted in the supplier withholding the supplies until they were paid. According to PTF 2017 [13] misappropriation of funds among other challenges added gravely to human misery due to lack of essential dugs. With implementation of proper budgeting and financing, these shortages could be reduced as KEMSA was readily willing to bring the procured drugs on time so long as they were assured of payments.
Lack of funds also resulted in the hospital quotation of their required drugs being reduced as all of the required drugs could not be afforded. The reduction of the quotation quantity resulted in the drugs ending before the target hospital catchment population was reached. Such shortages of drugs have also been reported in other counties such as Kericho, Kisumu and Busia [14]. The same article reported that the patients were paying more to get drugs from pharmacies and some sought alternative treatments from herbalists whom they considered cheaper.
The process ought to have been transparent. Bureaucracy brought about a potential for manipulation of contract awards and lack of fair competition, all of which created the perception in the population at large, that public expenditure is slow, ineffective, expensive and often corrupt [10]. Studies have shown that legal requirements lengthened the procurement process negatively affecting the consistency levels of obtaining pharmaceutical supplies [15].
Poor quantification was evident as the monthly reports did not contain accurate data and instead estimates were used at times due to lack of consistency in filling the daily activity register. Studies showed that it needed to involve accurate past consumption data with consideration of expected changes in morbidity patterns, seasonal factors, service levels, formulary changes or changes to prescribing patterns and patient attendance [11]. This could only be possible with the correct filling of the various daily forms used in storage and usage of pharmaceutical drugs.
The need for skilled personnel was evident as it would ensure effectiveness and proper quantification of the pharmaceutical drugs to be procured. It would eliminate small errors that could be easily avoided like over-estimation or under-estimation of the quantity required. The staff should be qualified with high professional and ethical standards and using sound procedures anchored in appropriate policies and regulations to ensure effectiveness. A study by Ombaka 2009 [2] revealed that pharmacists involved in hospital procurement of medicines, whether directly or indirectly, must be knowledgeable about medicines as well as the interacting issues and the many stakeholders who can potentially affect the process or who may have legal responsibility.
Transportation Problems were a crucial aspect and World Health Organization 2004 [16] stipulated that if there were problems with transportation and the drugs were delivered with e.g. leakages, they should be handed over to the delivery team with an Internal Drug Return (IDR) form. These discrepancies should be recorded in the remarks column in the stores requisition/delivery (issue) form.
Conclusion
The procurement procedure in NCRH was found to be in line with the guidelines set by the MOH and WHO. This procedure was found to face some challenges. The major challenge faced in the procurement of drugs in NCRH is inadequate funding and delay in paying the suppliers owing to bureaucracy required to acquire money from the county offices. Poor quantification also plays a crucial role. These challenges have adversely affected service delivery by delaying payment of supplier and that has led to delay in delivery of goods and drugs not being adequate to sustain the hospital population.
In the light of the above conclusions, the study recommends that transparency and accountability in the procurement process be ensured. There also needs to be equitable funding to the various county governments who also need to pay on time so as not to delay tenders. Checks and measures including thorough auditing needs to be provided to avoid misappropriation of funds. The hospital needs to have frequent reviews by the various departments eg: Pharmacy. They also need to ensure regular inventory control by the staff. Inventory management training by government is necessary to equip the staff with knowledge and skills on use and management of the various registers and forms for proper quantification.
References
- Dobler D, Burt D (1996) Purchasing and Supply Management. New York: McGraw Hill.
- Ombaka E (2009) Current Status Of Medicines Procurement. Am J Health-System Pharmacy.
- Food and Health Bureau (2013) Procurement and Supply of Pharmaceutical Products in Public and Private Medical Sectors. Pharmacological Review 37-43.
- World Health Organization (2002) Practical Guidelines on Pharmaceutical Procurement for Countries with Small Procurement Agencies. Manila, Philippines: World Health Organization.
- UNICEF (2010) Procurement Process for Pharmaceuticals. UNICEF.
- Hasan S, Wanyanga W (2010). Pharmaceutical Sector Profile: Kenya. Vienna: UNIDO.
- Romulo L (2014) Challenges of pharmaceutical regulation. Retrieved from EP Magazine.
- Quick J, Muziki S, Woldeyesus K, Fresle D, Grayston G, et al. (1999) Operational principles for good pharmaceutical procurement. Geneva: World Health Organization.
- Gabra M (2006) Session 6: Enhancing Pharmaceutical Procurement (powerpoint slides). Retrieved from.
- Oyamo EA, Mburu DK (2014) Effects of procurement processes on the distribution of pharmaceutical drugs in public hospitals in Kenya: A case of Mission for Essential Drugs and Supplies (MEDS). Prime J Social Science 721-732.
- World Health Organization (2012) Managing Procurement. Management Sciences for Health, chapter 18. Retrieved from World Health Organization.
- KEMSA (2017) KEMSA E-SERVICES. Retrieved from KEMSA.
- PTF (2017) Health Services. Retrieved from Partnership for Transparency: ptfund.org/health/
- Oketch AK (2016, March 14). Drug shortages takes toll on patients. Daily Nation, p: 28.
- Mutua DM (2013) factors Affecting Consistency in Supply of Pharmaceutical Products in Government Hospitals in Kenya: A case Study of Maragua District Hospital. International Institute for Science, Technology and Education (IISTE).
- World Health Organization (2004) Management of drugs at Health centre level. Brazzaville: WHO.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences